Abstract
Paravalvular abscess is a serious complication of infective endocarditis. The aortic valve and its adjacent ring are more susceptible to abscess formation and paravalvular extension than the mitral valve. A 15-years old patient with bicuspid aortic valve presented with staphylococcal tricuspid valve endocarditis complicated by para-aortic abscess that ruptured into the aortic sinus. We report the clinical, laboratory and echocardiographic features and treatment of this patient and conduct a literature review on this subject.
Paravalvular abscess is a serious complication of infective endocarditis. It is found in up to 40% of patients at surgery or autopsy.1) The aortic valve and its adjacent ring are more susceptible to abscess formation and paravalvular extension compared to the mitral valve.1)
We report a case of staphylococcal endocarditis involving the tricuspid valve complicated by a para-aortic abscess that ruptured into the aortic sinus diagnosed by trans-thoracic echocardiogram.
A 15-year-old male was admitted with a 12-day history of fever. He denied having traveled and contact with animals in the previous 3 months. He had experienced general malaise and headache for one week. His medical history was unremarkable, except left ankle trauma three months prior to presentation.
On admission, the blood pressure was 100/50 mmHg, pulse rate was 111/min, respiratory rate was 22/min and body temperature was 39.7℃. Auscultation revealed clear breathing sounds and a faint diastolic regurgitant murmur (1-2/6) at the left sternal border. In addition, reddish, painful left ankle edema, suggestive of cellulitis in addition to small erythematous macular lesions suggestive of Janeway lesions on the soles were present. Other physical examination findings were unremarkable. Electrocardiogram showed sinus tachycardia (107 beats/minute) without ST-T segment abnormality. Laboratory tests revealed a white blood cell count of 17,000/µL with elevated neutrophils (85.4%), a hemoglobin level of 13.2 g/dL, an elevated erythrocyte sedimentation rate (ESR) (23 mm/hour), a C-reactive protein 10.48 mg/dL and abnormal liver function with aspartate aminotransferase/alanine aminotransferase of 39/66 IU/L. There was no definite infiltration of the lung fields on chest radiograph. Transthoracic echocardiography (Fig. 1) revealed mild aortic regurgitation, bicuspid aortic valve and an oval echolucent area near the aortic valve adjacent to the right bicuspid aortic valve commissure with direct communication to the aortic sinus. An echolucent cavity was visible on the right side of the aortic valve annulus, and was thought to represent paravalvular ring abscess. A diastolic color flow jet into the abscess cavity indicated a communication with the aortic sinus. Examination of the tricuspid valve demonstrated a 1.4 cm mobile vegetation on the septal leaflet of the tricuspid valve associated with trivial tricuspid regurgitation. However, cardiac systolic function was normal with a 63% ejection fraction. Transesophageal echocardiography was performed on the fourth hospital day to further investigate the oval echolucent area near the aortic valve, which confirmed a bicuspid aortic valve along with a para-aortic abscess that communicated directly with the aortic sinus (Fig. 2). Computed tomography scan of the abdomen demonstrated multiple hypodense lesions in the spleen and the left kidney, considered to be abscesses secondary to septic emboli. Staphylococcus aureus (S. aureus), sensitive to ciprofloxacin, gentamicin, oxacillin, rifampin, teicoplanin, tetracycline, trimthoprim/sulfamethoxazole, and vancomycin, was isolated from peripheral blood cultures according to susceptibility test performed by the disc diffusion method. Ultimately, a diagnosis of acute staphylococcal endocarditis was made according to the Duke criteria, which included three positive blood culture results of S. aureus, the presence of vegetation on the tricuspid valve and para-aortic abscess as demonstrated by echocardiogram, in addition to clinical and laboratory features, such as left ankle cellulitis, fever, and Janeway lesion.
The patient was empirically treated with a combination of nafcillin (1 g/12 h) and gentamicin (60 mg/8 h) before blood culture results were known. Over the subsequent 48 hours, the patient's cellulitis improved markedly as evidenced by reducing erythema, swelling, and pain of the left ankle. Initially, the patient responded well, but after 7 days of intravenous antibiotic administration, laboratory results showed signs of increasing inflammation. A new rise in leukocytes counts from 9,400/µL to 10,000/µL, with elevated neutrophils from 73% to 79%, was observed. ESR increased from 21 mm/h to 24 mm/h at the time. Because the infection site was critical, we replaced intravenous nafcillin with intravenous vancomycin (1 g/12 h) and persisted with gentamicin (60 mg/8 h), with the suspicion of nafcillin resistance. Gentamicin was discontinued after the first 14 days of therapy.
Repeat transthoracic echocardiography demonstrated bicuspid aortic valve along with a persistent para-aortic abscess communicating with the aortic sinus. The size of tricuspid valve vegetation reduced from 15 mm to 7 mm after the first 14 days of antibiotic therapy. Repeated blood cultures were negative during antibiotic therapy. However, vancomycin therapy was discontinued after 21 days due to leukopenia (leukocyte count of 2,900/µL and neutrophil count of 14.4%). Antimicrobial therapy was discontinued because the patient showed no signs of active infection, and repeated blood cultures were negative. One week after discontinuing antibiotic therapy, blood analysis demonstrated a normal leukocyte count of 7,600/µL, a neutrophil of 66.6% and an ESR of 6 mm/h. Ultimately, the patient received 4 weeks of antibiotics with various regimens: nafcillin/gentamicin for 1 week followed by vancomycin/gentamicin for 1 week, followed by 2 weeks of vancomycin therapy. He was discharged in good condition. The patient made a recovery, as the tricuspid valve vegetation disappeared and the abscess cavity showed no significant change in size on the follow-up echocardiogram performed one month after discharge. There were no changes at 5-months follow-up.
Staphylococcal tricuspid valve endocarditis with para-aortic abscess in a patient with bicuspid aortic valve is rare. Tricuspid valve endocarditis is an uncommon condition in non-intravenous drug abusers. It is quite unusual for coagulase-negative staphylococci to cause endocarditis in a young, otherwise healthy person who was not an intravenous drug user, and who had no other risk factors predisposing to infective endocarditis.
Perivalvular abscess is an ominous development in patients with endocarditis, because it is associated with valvular and perivalvular destruction, leading to serious complications, such as heart block, pericarditis and acute valvular insufficiency.2) In patients with native valve endocarditis, the risk is increased when the valve is bicuspid. Ellis et al.3) described signs suggestive of the presence of perivalvular abscesses on transthoracic echocardiogram, e.g., rocking motion of the prosthetic valve, aneurysm of the sinus of Valsalva, increased subvalvular intraventricular septum thickness (≥13 mm), and thickening of the aortic root wall (≥10 mm). If any of these abnormalities is seen on transthoracic echocardiogram, or if there are clinical manifestations of abscess, transesophageal echocardiography is indicated.
It remains unclear whether detection of root abscess in the disease course of aortic valve endocarditis is an indication for surgery. Surgery in patients with active infection and perivalvular abscess carries a high risk of death, and is associated with significant risk of recurrent infection and periprosthetic leak postoperatively.4-8) Due to the patients' preoperative conditions secondary to sepsis, treatment of complex aortic valve endocarditis is a surgical challenge, with a reported mortality rate that ranges from 9% to 23%.9) Annular abscesses or intracardiac fistulae increase the technical difficulties and risks associated with surgical treatment.10) The long-term outcome of these patients has not been well defined.7)8) A small number of patients with periannular extension of infection or myocardial abscess may be treated successfully without surgical intervention.11)12) These patients potentially include those who have smaller (<1 cm) abscesses, and who do not have complications such as heart block, echocardiographic evidence of progression of abscess during therapy, valvular dehiscence or insufficiency. Cases of successful medical treatment of perivalvular abscess have been reported,11)13-15) but there have been no published series of consecutive patients and no comparison has been conducted with surgically treated patients. Surgical versus conservative treatment in patients with infective endocarditis and paravalvular abscess were associated with similar survival in another study.2) In a retrospective study of 233 cases of perivalvular abscess from 42 French hospitals over a 5-year period, medically treated patients who constituted 9% of the study population, had a survival rate similar to surgically treated patients.8) The findings of these study suggest that the main impetus for surgery is severe heart failure despite medical treatment, and that surgery may be deferred or avoided in patients whose heart failure is well controlled.2)8) In most patients, the abscess led to aortic pseudoaneurysm development secondary to cavitation and drainage into the aorta with control of the infection.6)16) The evolutional process was identical irrespective of the species of the infecting microorganism. Such patients should be monitored closely with serial transesophageal echocardiography, which should be repeated every 2, 4, and 8 weeks following completion of antimicrobial therapy. Jeang et al.17) report a case of aortic root abscess detected with magnetic resonance imaging and echocardiography, and the patient was successfully treated medically. The patient was alive with progressive reduction of abscess cavity size one year later. Our patient was treated medically for 4 weeks. For susceptible strains of S. aureus, vancomycin was more rapidly bactericidal than penicillin, nafcillin, and cefazolin.18) In a rabbit model of S. aureus endocarditis, sterilization of vegetations was more rapid with vancomycin.18) Combination of vancomycin and gentamicin resulted in more rapid clearing of organisms in animal models,19) and in some in vitro models of endocardial vegetations.20) In our patient, antimicrobial therapy was changed to vancomycin from nafcillin due to increasing inflammatory signs in the laboratory findings, suggestive of nafcillin resistance. Our patient has done well at one month. Although the abscess cavity is still present and showed no significant change in size, the patient has no current signs of infection. Infective endocarditis is a serious medical condition, and its management, especially regarding surgical intervention, should be individualized.
In summary, we report a case of staphylococcal infective endocarditis involving tricuspid valve complicated by para-aortic abscess in a 15-year-old man with bicuspid aortic valve. The patient received 4 weeks of antibiotics therapy and had a good quality of life at 5-month follow-up without surgical treatment. We will closely monitor the patient's long-term outcome.
Figures and Tables
Fig. 1
Short-axis view of the aortic valve on transthoracic echocardiography. A: a mobile vegetation (arrow) is noted at the base of septal leaflet of tricuspid valve. B: a large abscess cavity (arrow) communicating with the aortic sinus is seen in the para-aortic area.
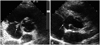
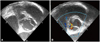
Fig. 2
Transesophageal echocardiography showed a bicuspid aortic valve with a large para-aortic abscess (1.6×2.1 cm) with direct communication to aortic sinus. A: the right side commissure of the bicuspid aortic valve is ruptured. B: color flow into the abscess cavity is visualized well during diastole.
References
1. Arnett EN, Roberts WC. Valve ring abscess in active infective endocarditis. Frequency, location, clues to clinical diagnosis from the study of 95 necropsy patients. Circulation. 1976. 54:140–145.
2. Chan KL. Early clinical course and long-term outcome of patients with infective endocarditis complicated by perivalvular abscess. CMAJ. 2002. 167:19–24.
3. Ellis SG, Goldstein J, Popp RL. Detection of endocarditis-associated perivalvular abscesses by two-dimensional echocardiography. J Am Coll Cardiol. 1985. 5:647–653.
4. Jault F, Gandjbakhch I, Chastre JC, et al. Prosthetic valve endocarditis with ring abscesses: surgical management and long-term results. J Thorac Cardiovasc Surg. 1993. 105:1106–1113.
5. Pansini S, di Summa M, Patane F, Forsennati PG, Serra M, Del Ponte S. Risk of recurrence after reoperation for prosthetic valve endocarditis. J Heart Valve Dis. 1997. 6:84–87.
6. Croft CH, Woodward W, Elliott A, Commerford PJ, Barnard CN, Beck W. Analysis of surgical versus medical therapy in active complicated native valve infective endocarditis. Am J Cardiol. 1983. 51:1650–1655.
7. Mullany CJ, Chua YL, Schaff HV, et al. Early and late survival after surgical treatment of culture-positive active endocarditis. Mayo Clin Proc. 1995. 70:517–525.
8. Choussat R, Thomas D, Isnard R, et al. Perivalvular abscesses associated with endocarditis: clinical features and prognostic factors of overall survival in a series of 233 cases. Eur Heart J. 1999. 20:232–241.
9. Muller LC, Chevtchik O, Bonatti JO, Muller S, Fille M, Laufer G. Treatment of destructive aortic valve endocarditis with the Freestyle Aortic Root Bioprosthesis. Ann Thorac Surg. 2003. 75:453–456.
10. Yankah AC, Klose H, Petzina R, Musci M, Siniawski H, Hetzer R. Surgical management of acute aortic root endocarditis with viable homograft: 13-year experience. Eur J Cardiothorac Surg. 2002. 21:260–267.
11. Kunis RL, Sherrid MV, McCabe JB, Grieco MH, Dwyer EM Jr. Successful medical therapy of mitral annular abscess complicating infective endocarditis. J Am Coll Cardiol. 1986. 7:953–955.
12. Park DW, Lee JH, Kang SJ, et al. Clinical characteristics of subaortic complications in patients with infective endocarditis of the aortic valve. Korean Circ J. 2004. 34:883–893.
13. Scanlan JG, Seward JB, Tajik AJ. Valve ring abscess in infective endocarditis: visualization with wide angle two dimensional echocardiography. Am J Cardiol. 1982. 49:1794–1800.
14. Tucker KJ, Johnson JA, Ong T, Mullen WL, Mailhot J. Medical management of prosthetic aortic valve endocarditis and aortic root abscess. Am Heart J. 1993. 125:1195–1197.
15. Byrd BF 3rd, Shelton ME, Wilson BH 3rd, Schillig S. Infective perivalvular abscess of the aortic ring: echocardiographic features and clinical course. Am J Cardiol. 1990. 66:102–105.
16. Sande MA, Courtney KB. Nafcillin-gentamicin synergism in experimental staphylococcal endocarditis. J Lab Clin Med. 1976. 88:118–124.
17. Jeang MK, Fuentes F, Gately A, Byrnes J, Lewis M. Aortic root abscess: initial experience using magnetic resonance imaging. Chest. 1986. 89:613–615.
18. Sande MA, Johnson ML. Antimicrobial therapy of experimental endocarditis caused by Staphylococcus aureus. J Infect Dis. 1975. 131:367–375.
19. Sande MA, Courtney KB. Nafcillin-gentamicin synergism in experimental staphylococcal endocarditis. J Lab Clin Med. 1976. 88:118–124.
20. Cha R, Brown WJ, Rybak MJ. Bactericidal activities of daptomycin, quinupristin-dalfopristin, and linezolid against vancomycin-resistant Staphylococcus aureus in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob Agents Chemother. 2003. 47:3960–3963.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download