Abstract
Coronary artery perforation (CAP) after percutaneous coronary intervention is a rare, but serious complication. It can cause cardiac tamponade, acute myocardial infarction or death. The treatments of CAP involve prolonged balloon inflation, emergent surgery, coil embolization, and implantation of covered stent. We have successfully performed the emergent microcoil embolization in a patient with uncontrolled Ellis grade 3 guidewire-induced CAP resulting in delayed cardiac tamponade. Contrasting our usual expectation, the 1-year follow-up angiography showed a patent flow at the embolized site.
Coronary artery perforation (CAP) which occurs either during or following percutaneuous coronary intervention (PCI) is an infrequent complication, but one of the most disastrous complications of this cardiovascular surgery procedure.1-9) It has been related to vessel wall penetration with guidewire, balloon overexpansion or rupture, atheroablative techniques, and stent implantation. Various management techniques have been successful. Some reports have shown that helical platinum microcoil embolization was used for CAP.10-15) Contrasting the usual expectation that an embolized artery will not show any blood flow, this report shows preserved normal blood flow at the embolization site on the 1 year follow-up angiography, following emergent lifesaving microcoil embolization, in a patient with uncontrolled Ellis grade 3 guidewire-induced CAP, resulting in cardiac tamponade. In the literature, there are no other similar case reports from a follow-up angiography after microcoil embolization, especially in a case such as the one presented herein, where the flow was maintained at the embolized site.
A 51-year-old male patient with a 30 pack-year current smoking presented with a recent onset of exertion chest pain. He had a free medical history. The treadmill test provoked severe chest pain, without ST changes on the electrocardiography (ECG), at stage 2 of the modified Bruce protocol. The patient underwent elective coronary angiography via the left femoral approach, which revealed a discrete critical narrowing of the middle left anterior descending artery (LAD), just distal to the first diagonal branch (D1) (Fig. 1A). A 7 Fr JL4 guide catheter (Cordis, Miami, FL, USA) was used to engage the left main coronary artery, and a 0.014" ATW™ guidewire (Cordis, Miami, FL, USA) was passed into the distal LAD, without difficulty. The proposed treatment was a cross-over stenting followed by provisional T stenting at that lesion site. Angioplasty was performed twice with the Ryujin™ Plus (TERUMO, Tokyo, Japan) 3.5×10 mm balloon at 6-atm (up to 3.5 mm) inflation. A Zotarolimus-Eluting stent (Endeavor Resolute, Medtronic, Minneapolis, MN, USA) 4.0×15 mm stent was deployed, with 12-atm (up to 4.08 mm) inflation. The repeat angiogram showed adequate deployment of the stent, but a jailed D1 (Fig. 1B). An attempt was made to pass into D1 using a 0.014" Choice™PT guidewire (Boston Scientific, Miami, FL, USA) through the instent. After initially inflating a Sprinter (Medtronic, Minneapolis, MN, USA) 3.0×15 mm semi-compliant balloon, up to 3.18 mm at the D1 ostium, 'kissing' balloon angioplasty, using a Sprinter 3.5×15 mm for the LAD and a Sprinter 3.0×15 mm for D1, was finally performed (Fig. 1C). The final angiographic result appeared good, without any complications (Fig. 1D). During observation in the intensive care unit, the patient grumbled about some chest tightness, but was hemodynamically stable. Three hours after PCI, he complained of more severe chest pain, dyspnea, tachypnea and heavy sweating. Moreover, his systolic blood pressure dropped to below 60 mmHg. An emergent echocardiogram was taken at the patient's bedside, under the suspicion of a cardiac tamponade, due to delayed extravasation after PCI. It showed a pericardial effusion of less than 10 mm, as well as the collapse of the right atrium. Emergency pericardiocentesis was performed and over 100 mL of bloody pericardial fluid was initially drained. The patient's vital signs returned to normal. Pericardial fluids were drained continuously and blood was transfused through the peripheral vein, with the expectation that it will stop the extravasation spontaneously. Considering the drainage rate of the pericardial fluid at over 200 mL/hr, a large perforation of the coronary artery at the stenting site was suspected. However, an emergent angiogram showed massive extravasation (Ellis Grade 3 coronary perforation) at the far distal area of D1 (Fig. 2A). An attempt was made to recross D1 and a Sprinter 3.0×15 mm semi-compliant coronary balloon with low pressure was inflated at the middle portion of D1. However, the perforation could not be sealed and continuous leakage occurred after the balloon's deflation, even after an inflation period of over 60 minutes. We attempted to occlude the D1 branch via a microcoil embolization to repair the perforation site completely. A MicroFerret® superselective catheter (Cook, Sandet, Denmark) was advanced superselectively into D1 at the level of the perforation. One 0.018-inch and 14.2-mm-long tapered microcoil (10×4 mm in diameter; Tornado; Cook, Bloomington, IN, USA) was quickly released, using the microcatheter's supporting guidewire (Transend® 300, Boston Scientific, Miami, FL, USA) as a pusher. A post-coil LAD arteriogram demonstrated the total occlusion of D1 and no further extravasation (Fig. 2B). On the following day, the post procedure echocardiogram revealed an ejection fraction of 60%, with mild hypokinesia on the apical lateral and apical anterior walls, and no pericardial effusion. The peak infarct size, as measured using serum biomarkers, was a troponin of 2.65 ng/mL and a creatinine phosphokinase-MB of 100.8 ng/mL. The patient was uneventful and discharged 5 days later in a stable condition. Three months after the PCI, he took the treadmill test and the echocardiogram. The treadmill test provoked chest pain at stage 5, with a 1 mm ST depression on the ECG, and the echocardiogram showed no interval change. For 12 months after PCI, the patient had several episodes of chest pain and dyspnea on exertion. He then underwent repeated coronary angiography. Angiographic findings of D1 showed a normal flow without delay (Fig. 2C). The treadmill test showed a negative finding.
CAP may range clinically from guidewire-related microperforations, resulting in minimal dye staining without haemodynamic consequences, to vessel rupture followed by active extravasations of blood and dye into the pericardial space, leading to tamponade and sudden haemodynamic collapse. It has been reported at variable rates, depending on the procedures performed, lesions treated and devices used. In the recent registries, perforation has been reported to occur in 0.17-1.5% of all patients undergoing PCI.9)16-18) The incidence of these complications has been increased by the use of debulking devices, such as high-speed rotational atherectomy and directional coronary atherectomy.2)3)5-7)17) Guidewire-induced perforation seems to be the most frequent cause of CAP-accounting for 20 to 68% of CAP incidents.2)3)5-9)16-18) In PCI of complex lesions, including chronic total occlusions and bifurcation lesions, the use of both hydrophilic and heavy-weight guidewires has also increased the frequency of this complication.2)4)7)9)17) A Choice™PT wire was used for crossing the D1 branch in this case. We suspected that the hydrophilic guidewire-induced damage occurred with the attempt to treat the jailed branch, using the 'kissing' balloon technique. This Choice™PT wire was coated with a highly lubricious hydrogel and was moderately stiff, compared with the new-generation guidewires. Due to this low coefficient of friction and easy distal migration,10)11)15)19) caution should be exercised in positioning the tip of the guidewire distally.
After the perforation diagnosis, treatment strategy varies, according to the clinical situation, i.e., the size of the perforation, the extent of contrast extravasation, and the hemodynamic status of the patients. The initial management involves the inflation of an angioplasty balloon proximal to or at the level of the perforation to seal the leak, and pericardioentesis can be performed if cardiac tamponade is present. Depending on the bleeding control, complete management modalities should be considered-from surgery to less invasive percutaneous techniques: covered stents/grafts or thrombus inducing therapies, such as polyvinyl alcohol, autologous blood or intracoronary bead injection. If a cardiac surgeon were unavailable, one might have to choose another option for controlling the patient's bleeding. Because a covered stent is bulky, has a large diameter (of over 3 mm), and is not easy to pass through the side hole of a stent in the parent artery, this device could not be used with the continuous bleeding from the perforation in this case. In the event that the perforation is in a small sized branch, the operator may consider excluding the whole branch by coiling, bead seeding, or implanting other thrombogenic agents directly into the branch. The use of the microcoil was very effective in the patient presented herein. In recent studies, the prevalence of microcoil embolization as a treatment option of CAP was 0.1-4%.1)8)17) It is not common but it may play an important role when traditional management for CAP has failed.
Microcoils have been used as permanent embolic agents for occlusion of cerebral aneurysms, gastrointestinal hemorrhage, and other peripheral vascular therapeutic maneuvers. They offer the advantage of precise placement into very distal locations. However, due to the permanent loss of the vessel lumen beyond the site of the microcoil placement and the subsequent infarction, their use in the coronary arteries should be limited to distal small-vessel perforation in life-threatening circumstances, in which no other options are readily available. The Tornade® embolization microcoils (Cook) have a soft platinum structure with synthetic fibers that maximize thrombogenicity. Moreover, their helical configuration maximizes the coil exposure to the cross-section of the lumen for the disruption of the flow. To allow proper adherence to the vessel wall, the coil size should be slightly (25%) larger than the target vessel diameter. This case describes the successful management of CAP, with the sacrifice of the D1 via coil embolization. Nevertheless, at the follow-up angiography, a patent blood flow was evident at the site where the microcoil was inserted. This case, therefore, is not only the first angiographic result of microcoil embolization presented to date, but also, particularly, the first example of maintenance of the blood flow at the embolized site, which was against our expectations of complete occlusion. Why did this happen? The best hypothesis is that, although a very large microcoil was inserted, initially inducing a thrombus, this thrombosis state could not be maintained due to inadequate adherence to the vessel under dual antiplatelet therapy.
The patient developed cardiac tamponade 3 hours after PCI. Although some perforations are quite evident in the catheterization laboratory, others remain initially occult, only to become clinically apparent hours after the procedure. Cardiac tamponade complications occur in 11-46% of patients experiencing CAP after PCI.1-4)7-9)16)17) Delayed tamponade comprised 20-60% of these tamponade complications,3)4)20) and was common in the perforations (e.g., ELLIS type I) induced by either guidewire or glycoprotein IIb/IIIa inhibitor use.16)20) Thus, this diagnosis should remain high on the list of differential diagnoses of post-PCI hypotension. In these cases, the diagnosis of CAP should be ruled out, especially if retroperitoneal bleeding is not present. Close observation is strongly advised in all PCI cases because, if not recognized, bleeding from CAP can rapidly lead to death from cardiac tamponade.
CAP is a rare, but disastrous complication of PCI. In selected cases of distal coronary perforations, microcoil embolization may be a reasonable alternative therapeutic approach. However, an obvious drawback of this approach is the permanent loss of the vessel lumen beyond the site of the microcoil placement and the subsequent infarction. Therefore, this approach should be limited either to life-threatening circumstances, in which no other options are readily available, or to the treatment of very distal perforations, where the amount of myocardium that is jeopardized is minimal. Despite a large, hemodynamically significant perforation, the successful management of CAP with the sacrifice of the D1 via coil embolization is described in this case. A notable, unexpected result was evident at the patient's follow-up one-year angiography, i.e., a patent blood flow existed at the site where the microcoil was inserted.
Figures and Tables
Fig. 1
Angiograms obtained during percutaneous coronary intervention. A: AP cranial view of the left anterior descending artery (LAD), showing a severe stenosis (black arrow) at the middle portion, just distal to the first diagonal branch (D1). B: view after 4.0×15 mm Zotarolimus-eluting stent was inserted at the middle LAD, showing a tight narrowing (white arrow) at the ostium of D1. C: kissing balloon angioplasty for LAD with a 3.5×15 mm Sprinter semi-compliant balloon and a 3.0×15 mm Sprinter balloon for D1. D: final angiogram.
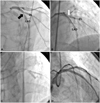
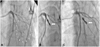
Fig. 2
An emergent and 1-year follow-up coronary angiogram. A: coronary artery perforation, evidenced by contrast leak into the pericardium from the distal end of the diagonal branch (D1) of left anterior descending artery (area marked by white triangles). B: embolization of D1 by helical microcoil (white arrow), with no evidence of contrast leak. C: showing surprising patent flow at the embolized site of D1 on the follow-up angiogram.
References
1. Gruberg L, Pinnow E, Flood R, et al. Incidence, management, and outcome of coronary artery perforation during percutaneous coronary intervention. Am J Cardiol. 2000. 86:680–682.
2. Dippel EJ, Kereiakes DJ, Tramuta DA, et al. Coronary perforation during percutaneous coronary intervention in the era of abciximab platelet glycoprotein IIb/IIIa blockade: an algorithm for percutaneous management. Catheter Cardiovasc Interv. 2001. 52:279–286.
3. Fukutomi T, Suzuki T, Popma JJ, et al. Early and late clinical outcomes following coronary perforation in patients undergoing percutaneous coronary intervention. Circ J. 2002. 66:349–356.
4. Gunning MG, Williams IL, Jewitt DE, Shah AM, Wainwright RJ, Thomas MR. Coronary artery perforation during percutaneous intervention: incidence and outcome. Heart. 2002. 88:495–498.
5. Fasseas P, Orford JL, Panetta CJ, et al. Incidence, correlates, management, and clinical outcome of coronary perforation: analysis of 16,298 procedures. Am Heart J. 2004. 147:140–145.
6. Stankovic G, Orlic D, Corvaja N, et al. Incidence, predictors, in-hospital, and late outcomes of coronary artery perforations. Am J Cardiol. 2004. 93:213–216.
7. Witzke CF, Martin-Herrero F, Clarke SC, Pomerantzev E, Palacios IF. The changing pattern of coronary perforation during percutaneous coronary intervention in the new device era. J Invasive Cardiol. 2004. 16:257–301.
8. Javaid A, Buch AN, Satler LF, et al. Management and outcomes of coronary artery perforation during percutaneous coronary intervention. Am J Cardiol. 2006. 98:911–914.
9. Kiernan TJ, Yan BP, Ruggiero N, et al. Coronary artery perforations in the contemporary interventional era. J Interv Cardiol. 2009. 22:350–353.
10. Gaxiola E, Browne KF. Coronary artery perforation repair using microcoil embolization. Cathet Cardiovasc Diagn. 1998. 43:474–476.
11. Aslam MS, Messersmith RN, Gilbert J, Lakier JB. Successful management of coronary artery perforation with helical platinum microcoil embolization. Catheter Cardiovasc Interv. 2000. 51:320–322.
12. Assali AR, Moustapha A, Sdringola S, Rihner M, Smalling RW. Successful treatment of coronary artery perforation in an abciximab-treated patient by microcoil embolization. Catheter Cardiovasc Interv. 2000. 51:487–489.
13. Banerjee S, Egdell R, Watkinson A, Greenbaum R. Coronary artery rupture treated with microcoil occlusion. Heart. 2001. 86:187.
14. Mahmud E, Douglas JS Jr. Coil embolization for successful treatment of perforation of chronically occluded proximal coronary artery. Catheter Cardiovasc Interv. 2001. 53:549–552.
15. Katsanos K, Patel S, Dourado R, Sabharwal T. Lifesaving embolization of coronary artery perforation. Cardiovasc Intervent Radiol. 2009. 32:1071–1074.
16. Georgiadou P, Karavolias G, Sbarouni E, Adamopoulos S, Malakos J, Voudris V. Coronary artery perforation in patients undergoing percutaneous coronary intervention: a single-centre report. Acute Card Care. 2009. 11:216–221.
17. Kini AS, Rafael OC, Sarkar K, et al. Changing outcomes and treatment strategies for wire induced coronary perforations in the era of bivalirudin use. Catheter Cardiovasc Interv. 2009. 74:700–707.
18. Ramana RK, Arab D, Joyal D, et al. Coronary artery perforation during percutaneous coronary intervention: incidence and outcomes in the new interventional era. J Invasive Cardiol. 2005. 17:603–605.
19. Wong CM, Kwong Mak GY, Chung DT. Distal coronary artery perforation resulting from the use of hydrophilic coated guidewire in tortuous vessels. Cathet Cardiovasc Diagn. 1998. 44:93–96.
20. Von Sohsten R, Kopistansky C, Cohen M, Kussmaul WG 3rd. Cardiac tamponade in the "new device" era: evaluation of 6999 consecutive percutaneous coronary interventions. Am Heart J. 2000. 140:279–283.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download