Abstract
Treatments of choice for cardiac implantable electronic device (CIED) infections are the removal of the entire CIED system, control of infection, and new device implantation. Occasionally, a complete CIED removal can not be performed for several reasons, such as very old age, severe comobidity, limited life expectancy, or refusal by a patient. We encountered a male patient who developed traumatic CIED infection five years after cardioverter-defibrillator implantation. An intravenous electrode could not be removed by a simple transvenous extraction procedure, and he refused surgical removal of the remnant electrode. After control of local infection, the tips of the electrode were separated and buried between muscles, and the wound was closed with a local flap. CIED infection did not recur for 12 months even without relying on long-term antimicrobial treatment.
Once cardiovascular implantable electronic device (CIED) infections encroach on a pulse generator pocket, the standard recommended treatment is removal of all the devices including the pulse generator and intravascular electrode(s).1-3) If the electrodes are not removed, the recurrence rate of local or systemic infection is extraordinarily high due to remnant hardwares.1)2)4-8) Although electrode removal may be easy for recent CIED implantations, it may be difficult in patients whose electrodes have been fixed with tight fibrosis following implantations performed several months or years earlier. Although many percutaneous devices have been developed to remove such immobile electrodes, these procedures involve significant risks including cardiac tamponade, hemothorax, pulmonary embolism, lead migration and death, even with experienced surgeons.4-8) When percutaneous removal of CIED system is impossible, open heart surgery should be performed to remove immobile electrodes but this surgery poses considerable risks.
Occasionally, electrode removal is impossible because the medical condition of the patient is unsuitable for a risky operation or the procedure is refused by a patient.9) Global experience on the management of patients with a remnant intravascular electrode in CIED infection is limited. Therefore, guidelines for such management have not been established yet. We describe a patient who refused percutaneous or surgical removal of an intravascular electrode and was treated effectively by repositioning the electrode to a safer area between the pectoralis major and minor muscles without relapse into local or systemic infection for 12 months. This is the first report of a successful repositioning of electrode procedure to our knowledge.
Although this is not a standard type of management for this condition, it can be considered a secondary treatment in these cases, since it addresses the needs of patients who have refused to remove remnant electrodes.
A 45-year-old male visited the out-patient department (OPD) with the primary complaint of skin erosion and pus discharge from a former implantable cardioverter-defibrillator (ICD) pocket.
He had been engaged in Christian missionary work in Novosibersk, Russia for the last 15 years and had a medical history of emergency surgery at a hospital in Novosibersk in May 1999 for primary closure of a stab injury, which penetrated the right ventricular free wall. He then suffered from dyspnea on exertion (New York Heart Association functional class II), palpitation and dizziness. The palpitation attacks developed and terminated suddenly, usually appearing once a month and lasting for a duration of 20 minutes to two hours. Sometimes they were relieved by deep inspiration and accompanied by chest discomfort, nausea and dizziness. Later on, palpitation attacks appeared more than once a day and loss of consciousness eventually developed after March 2004.
The patient visited the OPD on 15th June 2004, with a primary complaint of recurrent syncope. Two-dimensional echocardiography and a treadmill test revealed no evidence of organic heart disease. Holter monitoring showed non-sustained but symptomatic ventricular tachycardia (VT) with dizziness and lightheadedness. During an electrophysiological study, rapid ventricular pacing could easily and reproducibly induce VT, which sometimes degenerated into ventricular fibrillation (VF) with a syncope and hemodynamic compromise. Another session of electrophysiological study was performed with continuous intravascular infusion of an anti-arrhythmic agent, procainamide, without suppression of VT/VF induction. A single coil ventricular electrode was inserted into the apex of the right ventricle through a right subclavian puncture. The left upper chest area could not be used because there were previous operative scars and fibrosis (Fig. 1). An ICD pulse generator was inserted subcutaneously at the right anterior chest without complication on 12th July 2004.
Frequency of sustained or non-sustained VT attack decreased after oral medication with amiodarone and only five VT attacks were recorded between 2005 and 2008. All the attacks were successfully and appropriately converted to sinus rhythm and over the last year there were no VT/VF attacks.
The patient experienced blunt trauma at the area of the ICD pocket with a broomstick in May 2009 when he slipped on an icy road. The ICD pocket was swollen with hematoma, and the ICD pulse generator began to displace downward. The skin over the lower lateral angle of the pulse generator was thinning, and the pulse generator was exposed in July 2009. Although he self-sterilized the wound in Russia, pus discharge appeared two weeks after the erosion. He returned to Korea and visited the hospital.
Fever and other signs of systemic toxicity were absent and the patient presented with vague symptoms such as anorexia, fatigue, malaise and decreased functional capacity. A small cutaneous erosion with percutaneous exposure of the generator and pus discharge were evident upon inspection (Fig. 1). Three sets of blood culture were obtained, but he did not develop any appreciable fever over 38.3℃. Empiric vancomycin was initiated after the third set of blood culture.
The generator pocket was explored on the second day of admission. The generator was removed, meticulous debridement was performed, and cultures were obtained from the electrode tips and the pocket-site tissue. Removal of the electrode was also tried with conventional stylets without success. The causative organism did not grow in all cultures and therefore, systemic vancomycin was continued for 14 days. The wound was opened and wet dressing using betadine was performed twice a day. The second exploration, five days after the removal of the generator, revealed improved local infection and consequently, little debridement was required.
Follow-up transthoracic and transesophageal echocardiography (TEE) showed no evidence of infective endocarditis. The patient refused to remove the intravascular electrode after we explained the risks and benefits of open heart surgery to remove the electrode. The third exploration of the wound was done 10 days after the first exploration, and the wound was clear enough to be closed. The tips of the electrode were carefully displaced apart from the infected site, repositioned between the pectoralis major and minor muscles, and the skin was closed using a local flap without complication (Fig. 2). He also refused a second ICD implantation and was recommended to take oral amiodarone for the time being. He was discharged three days after wound closure, and visited the OPD daily for wound care. The stitches were removed 10 days after wound closure. First generation cephalosporin was given orally for 10 days after discharge. Regular OPD follow-up showed no recurrence of infection symptoms over the next 12 months. In addition, biomarkers, such as white blood cell count and differential count, erythrocyte sedimentation rate and C-reactive protein were normal. Palpitation or syncope did not develop over this period.
Recently an American Heart Association (AHA) committee updated the guidelines on CIED infections and their management.10) According to the guidelines, complete removal of all hardware, regardless of location (subcutaneous, transvenous or epicardial), is the recommended treatment for patients with established CIED infection because relapse rates of infection due to retained hardware are high.1)2)4-8) Established management for patients with CIED infections who are not candidates for device removal either by percutaneous or surgical methods has not been established. Often, these patients have a limited life expectancy or have refused device removal.9)10) Long-term antimicrobial suppressive therapy such as oral antibiotics for one to two years or more may be used in selected patients, but little is known about relapse rates, resistant organisms, safety profiles, patient compliance and financial expenses.9) In this case, we did not try long-term antibiotic treatment. Intravenous antibiotics were administered for 14 days while the patient was in the hospital and oral antibiotics were also administered for 10 more days after discharge (until the stitches were removed). There was no sign of recurrent infection for up to 12 months.
The length of time that antibiotics should be continued still needs to be resolved. According to AHA recommendations, blood cultures should be performed in every patient with suspected CIED infection. For patients with negative cultures like the patient discussed in this study, 10-14 days of antibiotics should be sufficient. For patients with positive cultures or prior antibiotic treatment, TEE is a useful test. For patients with negative TEE and non-S. aureus infection, 14 days of antibiotic treatment is sufficient. For patients with S. aureus infection, two to four weeks of antibiotic treatment are required. If vegetation is present in any of the cardiac valves, AHA guidelines for treatment of infective endocarditis should be followed. If complicated vegetation is found in intravascular leads, four to six weeks of antibiotic treatment are required. Realistically, an infected generator pocket will not close in a short period, and antibiotics should be continued until the stitches are removed.
A challenging issue is whether a new CIED is mandatory in patients who have had all hardware removed. In this case, a new ICD implantation could not be performed because, contrary to medical advice, the patient refused the implantation. Although there were no VT/VF episodes for the past year, he had five episodes of symptomatic VT/VF over the previous five years, and he definitely needed a new ICD implantation. A new, transvenous device implantation should not be done in an ipsilateral chest with CIED infection.10) Moreover, this patient had a remnant intravascular electrode through the right subclavian vein and an ipsilateral transvenous procedure might spread local infection directly into the blood stream. He also had previous emergency open-heart surgery scars on the anterior mid-chest, left lower chest, and at the left upper chest area where a new ICD should be placed (Fig. 1). The only option for a new ICD in this patient was epicardial ICD with general anesthesia and open chest surgery. Therefore, he decided not to have a new ICD and to take long-term oral amiodarone. But in most patients with CIED infection, each patient should be re-evaluated to determine whether a new CIED is necessary. One third to one half of patients in some study will not require a new CIED placement.2)
Another challenging issue is at what time a new CIED should be implanted in patients with CIED infection. The optimal timing of device replacement is unknown. If the infection is confined to the pocket, replacement after 72 hours of negative blood culture is generally accepted. However, if there is valve vegetation, implantation of a new CIED should be delayed 14 days from the first negative blood culture.2)5)11)
CIED infection is associated with substantial morbidity and mortality. Higher mortality is reported in patients with confirmed endocarditis and in those treated without device removal.11-15) All-cause mortality at six months with CIED infection was shown to be 18%, and variables associated with increased risk were systemic embolization, moderate to severe tricuspid regurgitation, abnormal right ventricular function and abnormal renal function.16)
Prevention of CIED infection is vital. Parenteral antibiotic prophylaxis, antiseptic preparation of the surgical site, a sterile technique, prevention of hematoma through meticulous bleeding control, irrigation with antimicrobial-containing solution for pocket cleansing, use of monofilament suture in the subcutaneous layer, pressure dressing for 12 to 24 hours after skin closure, and early follow-up after discharge all reduced CIED infection.10)17)18) Also, special attention should be paid to patients at greater risk of CIED infection. These risk factors include 1) renal dysfunction, 2) immunosuppression (corticosteroid use), 3) oral anticoagulation, 4) coexisting illness, 5) periprocedural factors (failure to administrate prophylactic antibiotic), 6) device revision-replacement, 7) operator experience, 8) the amount of indwelling hardware, and 9) the microbiology of the infectious organism.13)18-25)
Limited data on CIED infections are available in Korea. Choi et al.26) reported a long-term follow-up of 440 patients with permanent pacemaker implantation and only six CIED infections were reported. All the infections developed shortly after the implantation, and there were no difficulties in removing the entire CIED system. After the first ICD implantation in Korea, there was only one available ICD follow-up report on 28 patients, and the ICD infection did not appear.27)28) In Korea, CIED infection may increase because CIED implantation, revision/replacement, and implantation in patients with medical illness are increasing. Morbidity, mortality and cost are substantial in the treatment of CIED infection and the prevention of infection is of primary importance in CIED implantation.
Although the clinical course and pathophysiology of late-onset (six months after CIED implantation) CIED infections are not known, all devices should be removed in patients with established CIED. There were no signs of infection for 12 months in this patient. However, a chance of infection recurrence remains if the pathogen is dormant. Therefore, the treatment shown in this case should not be generalized and only considered as an auxiliary method in exceptional conditions such as very old age, severe co-morbidity, limited life expectancy, or refusal by a patient.
Figures and Tables
Fig. 1
A photograph of CIED infection. The ICD pocket was displaced downward and laterally, and the lower lateral edge was exposed by CIED erosion. The wounds at the central chest near the sternum, just below the left nipple, and at the left upper chest were made by the previous emergency operation: a primary closure of right ventricular free wall due to stab injury. CIED: cardiovascular implantable electronic device, ICD: implantable cardioverter-defibrillator.
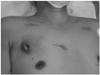
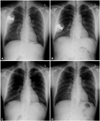
Fig. 2
Serial chest PA's. A: three days after the implantation of ICD on 15th July 2004. B: established CIED infection on 2nd September 2009. The ICD was displaced downward and laterally, and the intracardiac electrode was tethered. C: after ICD removal on 3rd September 2009. The wound was exposed, and the electrode could not be removed, therefore it was exposed as well. D: the tips of the ICD side were well separated and buried between the pectoralis major and minor muscles, and the wound was closed using a local flap on 12th September 2009.
References
1. Chua JD, Wilkoff BL, Lee I, Juratli N, Longworth DL, Gordon SM. Diagnosis and management of infections involving implantable electrophysiologic cardiac devices. Ann Intern Med. 2000. 133:604–608.
2. Sohail MR, Uslan DZ, Kahn AH, et al. Management and outcome of permanent and implantable cardioverter-defibrillator infections. J Am Coll Cardiol. 2007. 49:1851–1859.
3. Love CJ, Wilkoff BL, Byrd CL, et al. Recommendations for extraction of chronically implanted transvenous pacing and defibrillator leads; indications, facilities, training. Pacing Clin Electrophysiol. 2000. 23:544–551.
4. Baddour LM, Bettmann MA, Bolger AF, et al. Nonvalvular cardiovascular device-related infections. Circulation. 2003. 108:2015–2031.
5. Gaynor SL, Zierer A, Lawton JS, Gleva MJ, Damiano RJ Jr, Moon MR. Laser assistance for extraction of chronically implanted endocardial leads: infectious versus noninfectious indications. Pacing Clin Electrophysiol. 2006. 29:1352–1358.
6. Field ME, Jones SO, Epstein LM. How to select patients for lead extraction. Heart Rhythm. 2007. 4:978–985.
7. Chua JD, Wilkoff BL, Lee I, et al. Diagnosis and management of infections involving implantable electrophysiologic cardiac devices. Ann Intern Med. 2000. 133:604–608.
8. Sopeña B, Crespo M, Beiras X, et al. Individualized management of bacteraemia in patients with a permanent endocardial pacemaker. Clin Microbiol Infect. 2010. 16:274–280.
9. Baddour LM. Long-term suppressive antimicrobial therapy for intravascular device-related infections. Am J Med Sci. 2001. 322:209–212.
10. Baddour LM, Epstein AE, Erickson CC, et al. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation. 2010. 121:458–477.
11. Trappe HJ, Pfitzner P, Klein H, Wenzlaff P. Infections after cardioverter-defibrillator implantation: observations in 335 patients over 10 years. Br Heart J. 1995. 73:20–24.
12. Lai KK, Fontecchio SA. Infections associated with implantable cardioverter-defibrillators placed transvenously and via thoracotomies: epidemiology, infection control, and management. Clin Infect Dis. 1998. 27:265–269.
13. Gould PA, Krahn AD. Canadian Heart Rhythm Society Working Group on Device Advisories. Complications associated with implantable cardioverter-defibrillator replacement in response to device advisories. JAMA. 2006. 295:1907–1911.
14. O'Nunain S, Perez I, Roelke M, et al. The treatment of patients with infected implantable cardioverter-defibrillator systems. J Thorac Cardiovasc Surg. 1997. 113:121–129.
15. Sohail MR, Uslan DZ, Kahn AH, et al. Infective endocarditis complicating permanent pacemaker and implantable cardioverter-defibrillator infection. Mayo Clin Proc. 2008. 83:46–53.
16. Baman TS, Gupta SK, Valle JA, Yamata E. Risk factors for mortality in patients with cardiac device-related infection. Circ Arrhythm Electrophysiol. 2009. 2:129–134.
17. de Oliveira JC, Martinelli M, Nishioka SA, et al. Efficacy of antibiotic prophylaxis before the implantation of pacemakers and cardioverter-defibrillators: results of a large, prospective, randomized, double-blinded, placebo-controlled trial. Circ Arrhythm Electrophysiol. 2009. 2:29–34.
18. Lekkerkerker JC, van Nieuwkoop C, Trines SA, et al. Risk factors and time delay associated with cardiac device infections: Leiden device registry. Heart. 2009. 95:715–720.
19. Bloom H, Heeke B, Leon A, et al. Renal insufficiency and the risk of infection from pacemaker or defibrillator surgery. Pacing Clin Electrophysiol. 2006. 29:142–145.
20. Sohail MR, Uslan DZ, Khan AH, et al. Risk factor analysis of permanent pacemaker infection. Clin Infect Dis. 2007. 45:166–173.
21. Klug D, Balde M, Pavin D, et al. Risk factor related to infections of implanted pacemakers and cardioverter-defibrillators: results of a large prospective study. Circulation. 2007. 116:1349–1355.
22. Da Costa A, Kirkorian G, Cucherat M, et al. Antibiotic prophylaxis for permanent pacemaker implantation: a meta-analysis. Circulation. 1998. 97:1796–1801.
23. Al-Khatib SM, Lucas FL, Jollis JG, Malenka DJ, Wennberg DE. The relation between patients' outcome and the volume of cardioverter-defibrillator implantation procedures performed by physicians treating Medicare beneficiary. J Am Coll Cardiol. 2005. 46:1536–1540.
24. Chamis AL, Perteson GE, Cabell CH, et al. Staphylococcus aureus bacteremia in patients with permanent pacemakers or implantable cardioverter-defibrillators. Circulation. 2001. 104:1029–1033.
25. Kapa S, Hyberger L, Rea RF, Hayes DL. Complication risk with pulse generator change: implications when reacting to a device advisory or recall. Pacing Clin Electrophysiol. 2007. 30:730–733.
26. Choi HS, Rhee MY, Choi YJ, et al. Long-term follow-up of the patients with permanent antibradycardia pacemaker. Korean Circ J. 1998. 28:768–773.
27. Choi KJ, Lee CW, Kim JJ, Kim YH. Implantable cardioverter-defibrillator (ICD) therapy in a patient with the long QT syndrome. Korean Circ J. 1996. 26:1198–1203.
28. Kim DH, Kim SY, Lee KH, et al. Follow-up of a group of patients with automatic implantable defibrillator. Korean Circ J. 2005. 35:69–83.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download