Abstract
Background and Objectives
We aimed to investigate whether a large-sized Lasso catheter could increase the success rate of immediate complete pulmonary vein (PV) antral isolation and improve the outcome of catheter ablation in atrial fibrillation (AF) patients.
Subjects and Methods
This study included 107 consecutive patients (67 males, mean age: 57.8±9.7 years) who underwent PV mapping and ablation due to symptomatic drug-refractory AF. The first 43 patients underwent isolation of both ipsilateral PVs using the Carto-Merge 3 dimensional mapping system (group 1). The other 64 patients underwent isolation of both ipsilateral PVs using the same technique with a large-sized (a diameter of 30 to 35 mm) Lasso cathe-ter (group 2). When ipsilateral PVs did not show any potential after the initial circumferential ablation, we defined this as 'immediate complete antral isolation (ICAI)'. We compared the AF recurrence rate of both groups.
Results
There was no significant difference of the clinical characteristics between group 1 and group 2. All the patients were followed-up for 1 year. The ICAI rate of group 1 and group 2 was significantly different (21% vs. 78%, p<0.001), and the AF recurrence rates of group 1 and group 2 were also different (34.9% vs. 18.8%, p=0.042). Using multiple logistic regression analysis, the use of a large-sized Lasso catheter was a significant predictive factor for preventing recurrence (odds ratio: 0.489, 95% confidence interval: 0.136-0.927).
The pulmonary vein (PV) has been the main target for catheter ablation in patients with atrial fibrillation (AF) ever since it was identified as the major trigger for AF.1)2) Different techniques that target the PV include direct trigger ablation,3) segmental ostial PV isolation4-6) and circumferential PV isolation.7)8) The first two techniques are seldom used alone currently because of the high recurrence rate obtained. In circumferential PV isolation, the endpoint for ablation is the electrical disconnection of all PV potentials from the left atrium (LA). The PV can be isolated individually or as ipsilateral pairs, the so called 'en bloc' isolation, depending on the venous anatomy, the catheter stability and the operator's preference. In segmental ablation, to monitor complete PV isolation, one or two circular mapping catheters are placed at the ostia of the individual PV. This procedure was initially performed under fluoroscopic guidance, but it is now combined with a three dimensional (3D) electroanatomic mapping system (CARTO or NAVx). To the best of our knowledge, the feasibility and efficacy of using large-sized Lasso catheters, which encircle the whole ipsilateral superior and inferior PVs, in addition to using a 3D mapping system, have not yet been reported. In the present study, we aimed to investigate whether the large-sized Lasso catheter could affect the clinical outcomes of patients who undergo AF ablation using the Carto electroanatomic mapping system.
This study included 107 consecutive patients (67 males, mean age: 57.8±9.7 years) who underwent PV mapping and ablation between June 2005 and July 2007. All the patients had highly symptomatic, drug refractory paroxysmal or persistent episodes of AF. Patients who received stent placement or bypass surgery for significant coronary artery disease were excluded. We defined persistent AF as an episode of AF that lasted for over 7 days and required cardioversion to restore sinus rhythm. All the patients had been on treatment with at least one class I or III antiarrhythmic drug and had failed to maintain sinus rhythm before ablation. Each patient underwent transesophageal echocardiography to rule out the presence of left atrial thrombi before the procedure.
The patients were recruited in a prospective, non-randomized manner. The first 43 patients underwent transseptal isolation of both ipsilateral PVs using the 3D electroanatomic mapping system (group 1). The remaining 64 patients underwent isolation of both ipsilateral PVs using the same technique by placing a large-sized (diameter 30-35 mm) circular mapping catheter at the pulmonary antrums (group 2). The initial 20 cases for each group were excluded to rule out a learning curve effect. Written informed consent for the procedure was obtained from all patients and the study protocol was approved by the institutional review board at our medical center.
A spiral CT scan was obtained prior to the ablation procedure to evaluate the PV anatomy. The patients were given oral anticoagulation for at least 2 month before the procedure. This oral anticoagulation was discontinued 3 days before the procedure to achieve an international normalized ratio of prothrombin time that was less than 2.0. Amiodarone was discontinued at least 3 weeks before AF ablation and any other antiarrhythmic drugs were suspended for 1 week prior to the procedure.
During the procedure, the patient was given intravenous sedation with midazolam and fentanyl to achieve an appropriate level of consciousness. Continuous, non-invasive blood pressure, heart rate and oxygen saturation monitoring were performed throughout the procedure. The surface electrograms and bipolar endocardial electrograms were monitored and stored on a computer-based digital amplifier/recording system (Prucka CardioLab 7000 system, G.E. Medical systems, Milwaukee, WI, USA).
Three standard diagnostic catheters were positioned: a 6F hexapolar catheter at the His bundle area, a 7F decapolar catheter in the right atrium via the left femoral vein and a 7F decapolar catheter in the coronary sinus via the right jugular vein. A pigtail catheter was introduced into both pulmonary arteries via the right femoral vein, and the pulmonary venograms were obtained during the levo phase of both ipsilateral pulmonary artery angiographies.
Double transseptal catheterization was performed with an 8.5F SL1 sheath (St. Jude Medical, Inc., Minnetonka, MN, USA) using the Brockenbrough technique. An initial intravenous bolus of 5,000 IU heparin was given immediately after the transseptal puncture. Repeated doses of heparin were infused every 30 minutes to maintain an activated clotting time between 250 and 350 seconds.
Three-dimensional LA maps were reconstructed using a nonfluoroscopic navigation system (Carto XP, Biosense-Webster, Diamond Bar, CA, USA). Electroanatomic mapping was performed using an irrigated-tip catheter (Navistar Thermocool, Biosense-Webster, Diamond Bar, CA, USA). The Navistar catheter was placed inside each PV and then it was slowly drawn back to the LA for obtaining multiple PV points. Following that, the Navistar explored the entire LA, including the anterior wall, the posterior wall, the septal wall, the appendage and the mitral annular area. Over 150 points were sampled in each procedure. The CT images were transferred to the Carto system equipped with the image integration software (Carto-Merge™ Image Integration Module). The Carto-Merge software was used to superimpose the reconstructed 3D CT images on the real time LA electroanatomic map. The merge technique has been previously described.9) During the AF ablation, we set the fluoroscopy at 6 nGy/p with a pulse rate of 10/sec to reduce the radiation exposure.
Ablation was performed with the Navistar Thermocool catheter. Radiofrequency energy was applied with a power of 25 to 35 W and an irrigation rate of 17 to 40 mL/min. Radiofrequency energy was delivered in each spot for a maximum of 40 seconds or until the maximal local electrogram amplitude decreased by 80%. In group 1 patients, we performed circumferential antral ablation >5 mm away from the defined PV ostia after mapping with CartoMerge software (Fig. 1). After confirming that there was no anatomical gap on the 3D map, a steerable circular catheter with a diameter of either 25 mm or of variable size (Lasso, Biosense-Webster, Diamond Bar, CA, USA) was inserted into each of the PVs (Fig. 2). If the Lasso catheter showed no potential or a dissociated potential recorded in each PV, then we defined this as "immediate complete antral isolation (ICAI)" If the residual conduction between the PV and the LA was identified, then additional segmental ablation was performed on the previous ablation line, as guided by the earliest potentials detected on the standard-sized Lasso catheter. To ablate as close as possible on the previous ablation line, we preferred using the variable Lasso catheter adjusted to the diameter of the PV antrum.
For group 2 patients, a large-sized Lasso catheter (30-35 mm, Biosense-Webster) was placed at the beginning of the antral ablation (Fig. 3). This could encircle the entire ipsilateral PV in most cases. Circumferential PV isolation was guided by the large-sized Lasso catheter as well as the CartoMerge electroanatomic mapping. The isolation of each of the PVs was verified by putting the standard Lasso catheter (25 mm or variable size) into the ipsilateral superior and inferior PVs. ICAI was achieved in 78% of the patients. In the remaining patients, additional antral ablation, guided by a standard Lasso catheter, was needed in cases where the large size Lasso catheter did not fit correctly into the ipsilateral PV antrum.
The roof line ablation on the LA10) was performed in both groups after circumferential PV isolation. For patients with persistent AF, mitral isthmus ablation was added in both groups as previously described.11) The end point of ablation was defined as 1) the absence of PV potentials or dissociated PV potentials as documented with the standard size (25 mm) or variable size Lasso catheters within all four PVs and 2) bidirectional conduction block for the roof line and the mitral isthmus line.
After the procedure, anticoagulation with warfarin was restarted and continued for at least 3 months. Antiarrhythmic drugs were reused in 37 (86.0%) and 43 (72.9%) patients in group 1 and 2, respectively (p=0.110). Amiodarone was included for 9 (24.3%) and 10 (26.3) patients in group 1 and 2, respectively (p=0.843). All the patients were monitored by ECG during hospitalization. After being discharged, the patients underwent careful follow-up visits (one week after discharge followed by monthly visits) at the cardiology clinic. On each visit, patient symptoms and their surface ECG recordings were evaluated. The ambulatory 24-hour ECG monitoring was routinely examined at 3, 6 and 12 months, or whenever the patient exhibited symptoms. Any documented episode of symptomatic or asymptomatic AF that lasted for more than 30 seconds was considered to be a recurrence. AF recurrence within the first 3 months after ablation was not counted as a recurrence and those who did not show any evidence of tachycardia after 3 months of follow-up were considered as successful ablation. All the patients were followed-up for 1 year. Repeated ablation was not performed during this period.
Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) version 12.0 (SPSS Inc., Chicago, IL, USA). Continuous variables were expressed as means±SDs and they were compared using an unpaired Student's t-test. The categorical variables were compared with Fisher's exact test or the χ2 test, where appropriate. All the comparisons were two-sided, and p<0.05 were regarded as statistically significant. Multiple logistic regression analysis was performed to identify the independent predictors of AF recurrence.
The baseline characteristics are summarized in Table 1. Of the 107 study subjects, 67 (62.6%) were males (62.6%). The number of patients with diabetes and hypertension was 11 (10.3%) and 41 (38.3%), respectively. There was no significant difference for the clinical and procedural characteristics between group 1 and group 2. Non-fatal cardiac tamponade occurred in 2 patients (one in group 1 and the other in group 2, p=0.775).
The rate of successful ICAI showed a significant difference between group 1 and 2 (21% vs. 78%, respectively, p<0.001).
The total AF recurrence rate of group 1 and group 2 was 34.9% and 18.8%, respectively (Table 2). The recurrence rate was not significantly different between group 1 and group 2 for paroxysmal AF (p=0.210) and persistent AF (p=0.127).
The variables included in the multivariate analysis were LA size, left ventricular ejection fraction, gender, age, the type of AF and the use of a large-sized Lasso catheter. After multiple logistic regression analysis, the use of a large-sized Lasso catheter was significant as a predictive factor for preventing recurrence (odds ratio: 0.489, 95% confidence interval: 0.136-0.927).
In this study, we observed that using a large-sized Lasso catheter was an independent predictor of freedom from AF recurrence after catheter ablation. To our knowledge, this is the first report to investigate the feasibility and efficacy of using a large-sized Lasso catheter in addition to using 3D electroanatomic mapping.
Yamane et al.12) demonstrated the effectiveness of large-size Lasso catheters. The diameter of the Lasso catheter was 25-30 mm and they used it for antral isolation of individual PVs without the 3D mapping system. In contrast, we used a larger Lasso (35 mm in diameter) and performed the 'en bloc' ablation of the ipsilateral PVs.
PV antral isolation poses a difficulty in achieving a complete conduction block and requires a longer procedure time as compared to segmental ostial isolation. However, AF free survival was better for antral isolation than for segmental ostial isolation.13)14) Ouyang et al.7)8) have shown the feasibility of complete isolation around both ipsilateral PVs in combination with the 3D mapping system and the double-Lasso catheter. The reason for using the Lasso catheter in addition to the 3D mapping system is that it is easy to directly monitor the PV potentials and simultaneously observe conduction block when isolating the PV antrum. The limitation of using a large-sized Lasso catheter is that occasionally the contact of the catheter is not good enough because of the anatomic variance around the PV antrum. The AF recurrence rate of our study was slightly lower than that of a previous study.7) The addition of the roof and mitral isthmus line might have contributed to the lower rate of AF relapse in our study.
In the present study, the rate of AF recurrence was lower in group 2 than in group 1 and the large-sized Lasso catheter was a significant predictor for AF recurrence using multivariate analysis. Complete electrical isolation of the PVs has a significant impact on the long-term efficacy of the procedure15) and the recovered PV conduction after the AF ablation is a dominant factor for AF recurrence.16) In group 1, only 21% of the patients achieved ICAI by 3D-guided antral ablation alone and the rest of them needed additional antral ablation using the standard Lasso catheter. In contrast, we could achieve ICAI after initial antral isolation in 78% of the group 2 patients. In both groups, we tried to deliver radiofrequency energy on the previous line as close as possible, but in some cases additional ablation should have been performed inside the PVs. Although we did not check the PV reconnection in the relapsed patients by a redo procedure, we assumed that there may be some differences in the recovered PV conduction between the two groups. Additional linear lesions might also have contributed to such ambiguous results.
First, this study has limitations because of its small sample size and non-randomized design. Second, we performed 24-hour Holter monitoring because 7-day Holter monitoring or a transtelephonic recording system was not available. Thus, we may have underestimated the asymptomatic recurrence of AF. However, this limitation would have equally affected both treatment groups. Further investigation will be required to determine whether such an approach will have practical implications.
The combination method of using the Carto-Merge 3D electroanatomic mapping system together with a large sized Lasso catheter for AF ablation seem to be more effective than 3D mapping only for the prevention of AF recurrence. A large sized Lasso catheter plays an important role to achieve ICAI. The reason for using the Lasso catheter in addition to the 3D mapping system is that it is easy to directly monitor the PV potentials and simultaneously observe conduction block when isolating the PV antrum.
Figures and Tables
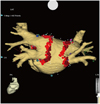 | Fig. 1Three-dimensional mapping of the Carto-Merge on the right anterior oblique view. Circumferential ablation around both ipsilateral veins is shown by the red dots. |
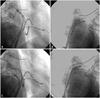 | Fig. 2Fluoroscopic image confirming the isolation of each pulmonary vein. A variable-sized Lasso catheter was used to verify conduction block between the pulmonary vein and the left atrium. Dashed lines are schemes of the ipsilateral pulmonary vein. A: right superior pulmonary vein. B: left superior pulmonary vein. C: right inferior pulmonary vein. D: left inferior pulmonary vein. |
 | Fig. 3Fluoroscopic image in which a large-sized Lasso catheter (35 mm) was used. In group 2 patients, a large-sized Lasso catheter was placed at the beginning of the antral ablation. Dashed lines are schemes of ipsilateral pulmonary vein. A: right pulmonary vein. B: left pulmonary vein. |
References
1. Haissaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998. 339:659–666.
2. Nam KB, Choi KJ, Park DW, Kim J, Rhee KS, Kim YH. Electrophysiological characteristics of arterially-perfused canine pulmonary veins: role of the delayed afterdepolarization-induced triggered activity. Korean Circ J. 2005. 35:643–648.
3. Jais P, Haissaguerre M, Shah DC, et al. A focal source of atrial fibrillation treated by discrete radiofrequency ablation. Circulation. 1997. 95:572–576.
4. Haissaguerre M, Jais P, Shah DC, et al. Electrophysiological end point for catheter ablation of atrial fibrillation initiated from multiple pulmonary venous foci. Circulation. 2000. 101:1409–1417.
5. Chen SA, Tai CT, Tsai CF, Hsieh MH, Ding YA, Chang MS. Radiofrequency catheter ablation of atrial fibrillation initiated by pulmonary vein ectopic beats. J Cardiovasc Electrophysiol. 2000. 11:218–227.
6. Oral H, Knight BP, Ozaydin M, et al. Segmental ostial ablation to isolate the pulmonary veins during atrial fibrillation: feasibility and mechanistic insights. Circulation. 2002. 106:1256–1262.
7. Ouyang F, Bansch D, Ernst S, et al. Complete isolation of left atrium surrounding the pulmonary veins: new insights from the double-Lasso technique in paroxysmal atrial fibrillation. Circulation. 2004. 110:2090–2096.
8. Ouyang F, Ernst S, Chun J, et al. Electrophysiological findings during ablation of persistent atrial fibrillation with electroanatomic mapping and double Lasso catheter technique. Circulation. 2005. 112:3038–3048.
9. Dong J, Dickfeld T, Dalal D, et al. Initial experience in the use of integrated electroanatomic mapping with three-dimensional MR/CT images to guide catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2006. 17:459–466.
10. Hocini M, Jais P, Sanders P, et al. Techniques, evaluation, and consequences of linear block at the left atrial roof in paroxysmal atrial fibrillation: a prospective randomized study. Circulation. 2005. 112:3688–3696.
11. Jais P, Hocini M, Hsu LF, et al. Technique and results of linear ablation at the mitral isthmus. Circulation. 2004. 110:2996–3002.
12. Yamane T, Date T, Kanzaki Y, et al. Segmental pulmonary vein antrum isolation using the "large-size" lasso catheter in patients with atrial fibrillation. Circ J. 2007. 71:753–760.
13. Oral H, Scharf C, Chugh A, et al. Catheter ablation for paroxysmal atrial fibrillation: segmental pulmonary vein ostial ablation versus left atrial ablation. Circulation. 2003. 108:2355–2360.
14. Arentz T, Weber R, Burkle G, et al. Small or large isolation areas around the pulmonary veins for the treatment of atrial fibrillation? Results from a prospective randomized study. Circulation. 2007. 115:3057–3063.
15. Cheema A, Dong J, Dalal D, et al. Long-term safety and efficacy of circumferential ablation with pulmonary vein isolation. J Cardiovasc Electrophysiol. 2006. 17:1080–1085.
16. Ouyang F, Antz M, Ernst S, et al. Recovered pulmonary vein conduction as a dominant factor for recurrent atrial tachyarrhythmias after complete circular isolation of the pulmonary veins: lessons from double Lasso technique. Circulation. 2005. 111:127–135.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download