Abstract
Background and Objectives
In addition to diagnostic criteria, a broad range of nonspecific clinical features can be found in patients with Kawasaki disease. This lack of specificity may cause confusion with other febrile illnesses and delay the diagnosis of Kawasaki disease. The purpose of this study is to describe common associated symptoms and their clinical significances in children affected with Kawasaki disease.
Subjects and Methods
As a retrospective study, we reviewed the medical records of 121 children who were treated for Kawasaki disease at Presbyterian medical center from January 2005 to June 2010. All clinical symptoms, laboratory data and echocardiographic findings in patients with KD were collected and analyzed.
Results
We found that there are 9 associated symptoms, namely cough, rhinorrhea, sputum, abdominal pain, vomiting, diarrhea, arthralgia, headache and seizure, which occur in patients with Kawasaki disease. Among the study group, there were only 32 children with no associated symptoms (26.4%). Patients with abdominal pain and headache had an older mean age than those without such symptoms. The incidence of seizure was significantly higher in incomplete Kawasaki disease patients compared with those with complete Kawasaki disease. Vomiting was highly associated with IVIG non-responder group.
Go to : 
Kawasaki disease (KD) is an acute, febrile, multi-systemic vasculitis of unknown etiology, first reported by Kawasaki et al.1) KD is known as self-limiting, even in the absence of specific therapy.2) However, coronary artery aneurysm (CAA) or ectasia develop in 15-25% of untreated patients with the disease. These sequelae may lead to myocardial infarction, ischemic heart disease or sudden death.3-5) Early detection and prompt initiation of therapy with high dose intravenous immunoglobulin (IVIG) plus aspirin can reduce the incidence of serious coronary artery complications.6-8) Therefore, accurate and timely diagnosis of KD is critical. Because of the absence of a specific diagnostic tests or pathognomonic clinical features, physicians must rely upon the presence of specific clinical criteria and laboratory data that support the diagnosis of KD, while excluding other illnesses that could mimic the disease. However, in many cases, these clinical criteria for KD may not all present on any given day and not all patients with KD have all specific clinical features. Moreover, some patients often have other associated symptoms which may delay KD diagnosis and thus increase the incidence of coronary artery complications.
Few data have been reported on common associated symptoms. The aim of this study was to describe clinical feature, especially common associated symptoms and its clinical significances in children affected with KD.
Go to : 
We reviewed the medical records at Presbyterian medical center, retrospectively, of all patients with KD from January 2005 to June 2010. Clinical profiles, initial laboratory data, echocardiographic findings and follow-up data were collected from each medical record. All clinical symptoms were initially obtained both from observation and interview of primary caregivers.
The diagnoses of KD was confirmed by a pediatrician, based on the American Heart Association and the Japanese Ministry of Health and Welfare criteria.9-11) This diagnosis included the presence of 5 days of fever and ≥4 of the following 5 principal clinical features:
1) Bilateral bulbar conjunctival injection without exudate
2) Changes in lips and oral cavity
3) Polymorphous exanthema
4) Changes in extremities
5) Cervical lymphadenopathy
Patients with fever for 5 days and <4 principal features can be diagnosed as having KD when coronary artery lesion (CAL) is detected by 2-dimensional (2D) echocardiography. Diagnosis of incomplete KD (IKD) was made when clinical manifestations did not fulfill the diagnostic criteria and other diagnoses were excluded. Symptoms frequently associated with KD and elevated inflammatory indices during the acute phase were considered as supportive findings for the diagnosis of IKD.9)11) IVIG-responder was defined as patients who received a single dose of IVIG treatment and their fever subsided within 48 hours and inflammatory indices showed marked improvement. IVIG-non-responder was defined as patients who needed a second dose of IVIG because of a reappearance of fever within 48 hours after initial IVIG treatment. All patients were treated with both an IVIG (2 g/kg) and aspirin (80-100 mg/kg/day) during the acute phase of KD.
All patients underwent 2D echocardiography of the coronary arteries. Normal internal diameters of the coronary arteries were defined as 2.5 mm or less, in patient with body surface area (BSA) of less than 0.5 m2, 3 mm or less in those with BSA between 0.5 and 1.0 m2, and 4 mm or less in those of BSA of more than 1.0 m2. Coronary artery dilatation was determined to exist when coronary artery size was larger than normal, but with no apparent segmental aneurysm. CAA was defined when the internal diameter of a measured segment was >1.5 times that of an adjacent segment.12)
Data were analyzed using the frequency analysis, descriptive statistics, chi-square test, Fisher's Exact test, independent t-test, and binary logistic regression. A p<0.05 was considered statically significant. All analyses were conducted with Statistical Package for the Social Sciences version 18.0.
Go to : 
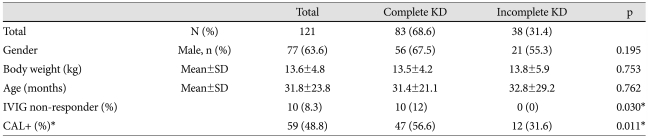
A total of 121 children with KD were included in the study. The mean age at diagnosis was 31.9 months (range: 3-142 months). The average body weight was 13.61 kg (range: 5.9-35.5 kg). There were 77 boys (63.6%) and 44 girls (36.4%). Among the 121 patients, 38 (31.4%) were IKD. IVIG-non-responder group contained 10 patients (8.3%), while IVIG-responder group contained 111 patients (91.7%). Of the 121 patients, 59 had CAL (48.8%), including 7 CAA (5.8%). Incidence of CAL in IVIG-non-responder was significantly higher than in IVIG-responder (80% vs. 45.9%, p=0.039).
All patients had a fever at the time of diagnosis. Among the five cardinal symptoms, conjunctival injection (84.3%) occurred most frequently, followed by polymorphous rash (81.0%) and oral mucosal changes (81.0%). Changes of extremities (58.7%) and cervical lymphadenopathy (51.2%) occurred less frequently than did other cardinal symptoms. In addition to major symptoms, there were 9 nonspecific associated symptoms that occurred in study subjects: cough was reported in 56 patients (46.3%), rhinorrhea in 37 patients (30.6%), sputum in 18 patients (14.9%), abdominal pain in 15 patients (12.4%), vomiting in 22 patients (18.2%), diarrhea in 19 patients (15.7%), arthralgia in 15 patients (12.4%), headache in 3 patients (2.5%), and seizure in 6 patients (5.0%). There were 7 symptoms that occurred in more than 10% of study subjects. One or more respiratory symptoms (cough, rhinorrhea, sputum) were present in 66 patients (54.5%). Forty-one (41) patients (33.9%) had at least gastrointestinal symptoms (vomiting, diarrhea or abdominal pain). There were 32 (26.4%) children with no associated symptoms.
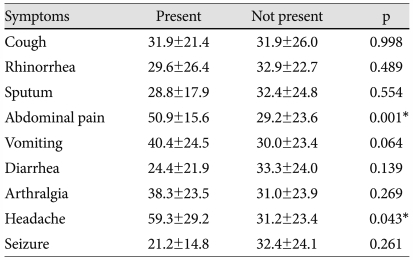
We analyzed the relationship of the common associated symptoms to patient age. Patients with abdominal pain and headache symptoms had an older mean age than those without such symptoms (p=0.001 and p=0.043, respectively) (Table 2). The incidence of associated symptoms did not significantly differ according to gender or days of illness at diagnosis.
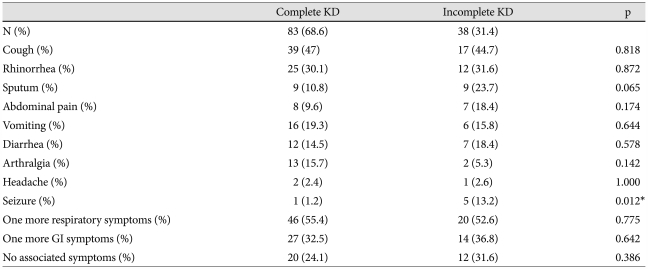
Compared with IKD, the incidence of major symptoms that were using classic diagnostic criteria were higher in complete KD. Among the associated symptoms, the incidence of seizure was significantly higher in IKD patients (Table 3). The incidence of CAL and IVIG-non-responder was significantly higher in complete KD than IKD (Table 1). In laboratory data, acute inflammatory indices (white blood cell count, erythrocyte sedimentation rate, C-reactive protein), aspartate aminotransferase and alanine aminotransferase were more elevated in complete KD patients, but not significant between both groups. However, the incidence of sterile pyuria was significantly higher in complete KD patients {complete KD vs. IKD: 39 patients (47%) vs. 10 patients (26.3%), p=0.032}. There were no significant differences between complete and IKD patients in terms of gender, mean age, and average body weight.
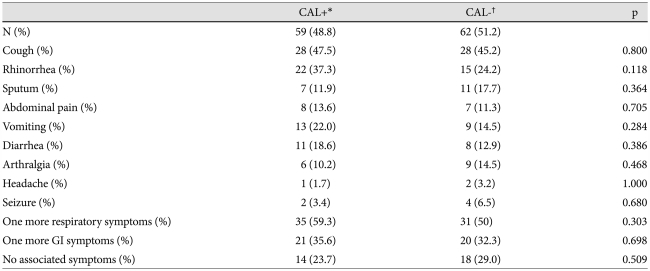
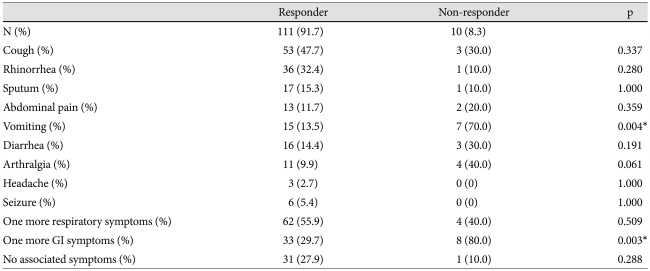
We also explored whether clinical symptoms were associated with development of CAL and IVIG responsiveness. The results indicated that there were no statistical differences in the incidence of clinical symptoms (major and minor symptoms) between the KD patient groups with or without CAL. There were, however, 45 (76.3%) patients who had one or more associated symptoms among the KD patients with CAL (Table 4). Interestingly, only the symptom of vomiting was significantly associated with the IVIG non-responder group (Table 5).
Go to : 
After the first case of KD was reported by Dr. Kawasaki in 1967, it has become the leading cause of acquired heart disease during childhood in many countries. Despite extensive investigation, the etiology of KD remains unknown, so the diagnosis depends on non-specific clinical signs rather than a definitive laboratory test. In addition to the diagnostic criteria, a broad range of non-specific clinical features are associated with KD, including irritability, uveitis, aseptic meningitis, seizure, severe lethargy, semicoma, cough, rhinorrhea, abdominal pain, vomiting, diarrhea, hepatic dysfunction, gallbladder hydrops, parotitis, urethritis, arthralgia.2)9)13-15)
We found that associated symptoms such as cough, rhinorrhea, sputum, abdominal pain, vomiting, diarrhea, arthralgia, headache and seizure commonly occur in patients with KD. Although, we can't completely rule out the possibility that associated symptoms result from concurrent infections we suspect that these symptoms may reflect diffuse vasculitis or be the sequelae of an infectious trigger of KD.
Few previous reports quantify the prevalence of associated symptoms in KD. In an early retrospective review, Hicks and Melish14)15) described their experience with KD, reporting that sterile pyuria reflecting urethritis is found in about 75% of patients. Additionally, arthritis occurred in 30% of KD patients and about one third of patients with arthritis have onset in the first 10 days of illness. In about 30% of the patients, gastrointestinal complaints, including abdominal pain, diarrhea and nausea, presented. CNS involvement with severe lethargy, semicoma, and aseptic meningitis occurs in 25% of patients. Also, obstructive jaundice and acute hydrops of the gallbladder are seen in about 5% of the patients. Park et al.16) reported that many school-aged patients with KD have incomplete presentation and occasionally show additional symptoms including abdominal pain, respiratory symptoms, headache, vomiting and arthralgia. Cho et al.17) reported that respiratory symptoms were observed in 69% of KD patients but the association between a respiratory virus and KD was not found. Baker et al.18) reported that nonspecific symptoms commonly occur in children with KD, finding that nine symptoms occurred in more than 10% of study subjects during the early phase of disease. They also noted that patients with irritability and rhinorrhea had a younger mean age than did those without these symptoms. In addition, the patients with symptoms of vomiting, abdominal pain and arthralgia were older than those without such symptoms. These results are similar to those that we found; in our study, seven common associated symptoms occurred in more than 10% of patients with KD. Also, patients with headache and abdominal pain were older than those without such symptoms. But, subjective symptoms such as abdominal pain, arthralgia and headache seem to become more prevalent with higher patient age, because older patients have better ability to understand and articulate their symptoms. Thus, further observations and studies are required to get more precise result.
There have been many studies done to determine factors related to the development of CAL and IVIG responsiveness over several years, however there has been little research into trying to ascertain the relationship between either occurrence of CAL and symptoms in KD, and IVIG responsiveness and symptoms in KD. This study tried to find out whether there were any relations between the existence of the associated symptoms in children with KD and the occurrence of CAL, between the number of symptoms in KD and the occurrence of CAL, between the existence of the associated symptoms in KD and IVIG-responsiveness, and between the number of symptoms in KD and IVIG-responsiveness through statistical analysis. There were no meaningful statistical correlations except for a few more vomiting symptoms in IVIG-non-responders (p=0.004). Despite this correlation, we do not have a clear explanation for why this occurs, but we think some inflammatory mediators that associated with IVIG-non-responders may affect the development of such symptoms. Because this study was implemented in only one institution, more studies are needed to statistically validate this study.
Although associated symptoms do not contribute to the principal criteria for diagnosis and treatment of KD, these symptoms may cause confusion with other common febrile illnesses and delay the diagnosis of KD. It should be emphasized that the clinical signs of KD are often not all present at any one point in time and that nonspecific associated symptoms might occur in KD patients occasionally. The presence of these symptoms should not cause the physician to discount the possibility of KD in patients with characteristic clinical findings.
The limitations of this study include its retrospective nature and relatively small sample size. Our study is restricted to a single center experience and thus cannot represent general characteristics of all KD patients, therefore a large multi-center-based investigation needs to take place in order to get a more precise result.
In conclusion, to decrease the incidence of serious coronary artery complications due to delayed diagnosis of Kawasaki disease, clinicians should certainly be aware of all of the manifestation of KD and the possibility of associated symptoms. Furthermore, careful evaluation of clinical findings and early study of echocardiography are needed.
Go to : 
References
1. Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Arerugi. 1967; 16:178–222. PMID: 6062087.
2. Burns JC, Glodé MP. Kawasaki syndrome. Lancet. 2004; 364:533–544. PMID: 15302199.
3. Kato H, Sugimura T, Akagi T, et al. Long-term consequences of Kawasaki disease: a 10- to 21-year follow-up study of 594 patients. Circulation. 1996; 94:1379–1385. PMID: 8822996.
4. Dajani AS, Taubert KA, Gerber MA, et al. Diagnosis and therapy of Kawasaki disease in children. Circulation. 1993; 87:1776–1780. PMID: 8491037.
5. Lee SY, Gwon HC, Park SW, et al. Acute myocardial infarction in young patient probably due to Kawasaki disease. Korean Circ J. 2001; 31:119–124.
6. Newburger JW, Takahashi M, Burns JC, et al. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med. 1986; 315:341–347. PMID: 2426590.
7. Newburger JW, Takahashi M, Beiser AS, et al. A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. N Engl J Med. 1991; 324:1633–1639. PMID: 1709446.
8. Terai M, Shulman ST. Prevalence of coronary artery abnormalities in Kawasaki disease is highly dependent on gamma globulin dose but independent of salicylate dose. J Pediatr. 1997; 131:888–893. PMID: 9427895.
9. Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004; 110:2747–2771. PMID: 15505111.
10. Research Committe on Kawasaki Disease. Report of Subcommittee on Standardization of Diagnostic Criteria and Reporting of Coronary Artery Lesions in Kawasaki Disease. 1984. Tokyo, Japan: Ministry of Health and welfare.
11. Ayusawa M, Sonobe T, Uemura S, et al. Revision of diagnostic guidelines for Kawasaki disease (the 5th revised edition). Pediatr Int. 2005; 47:232–234. PMID: 15771703.
12. Nakano H, Ueda K, Saito A, Nojima K. Repeated quantitative angiograms in coronary arterial aneurysm in Kawasaki disease. Am J Cardiol. 1985; 56:846–851. PMID: 4061324.
13. Do HJ, Baek JG, Kim HJ, et al. Kawasaki disease presenting as parotitis in a 3-month-old infant. Korean Circ J. 2009; 39:502–504. PMID: 19997548.
14. Hicks RV, Melish ME. Kawasaki syndrome. Pediatr Clin North Am. 1986; 33:1151–1175. PMID: 3532006.
15. Melish ME, Hicks RV. Kawasaki syndrome: clinical features. Pathophysiology, etiology and therapy. J Rheumatol Suppl. 1990; 24:2–10. PMID: 1700121.
16. Park EY, Kim JH, Kim HS, Sohn SJ. Clinical features of Kawasaki disease in school-aged children. Korean J Pediatr. 2007; 50:292–297.
17. Cho EY, Eun BW, Kim NH, et al. Association between Kawasaki disease and acute respiratory viral infections. Korean J Pediatr. 2009; 52:1241–1248.
18. Baker AL, Lu M, Minich LL, et al. Associated symptoms in the ten days before diagnosis of Kawasaki disease. J Pediatr. 2009; 154:592–595. PMID: 19038400.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download