Abstract
Sparganosis is caused by a larval tapeworm of the genus Spirometra, which commonly invades subcutaneous tissue, but less frequently invades muscle, intestines, spinal cord, and the peritoneopleural cavity. The authors managed a female patient who presented with a recurrent pericardiopleural effusion and peripheral eosinophilia. The anti-sparganum-specific IgG serum level was significantly higher than normal control levels. In this patient, sparganosis was caused by the ingestion of raw frogs in an effort to control her thyroid disease. The recurrent pericardiopleural effusion and peripheral eosinophilia were controlled by 3 consecutive doses of praziquantel (75 mg/kg/day). The patient is doing well 4 years after presentation. Sparganosis should be considered a rare, but possible cause of recurrent pericardial effusion and peripheral eosinophilia. Immunoserologic testing using enzyme linked immunosorbent assays can be helpful in diagnosing human sparganosis, especially in cases without a subcutaneous lump or mass. Praziquantel is an alternative treatment for sparganosis in surgically-unresectable cases.
Sparganosis is an infectious disease caused by the plerocercoid larvae of various diphyllobothroid tapeworms of the genus Spirometra, such as S. mansoni, S. mansonoides, S. erinacei, S. ranarum, S. decipiens, S. houghtoni, and S. proliferum.1)2) The common clinical manifestation of sparganosis is a subcutaneous lump or mass in the abdominal wall, scrotum, lower extremity, or chest wall. The sparganum can invade muscle, intestines, breast tissue, eyes, spinal cord, and rarely, the peritoneopleural cavity.3) Pleural effusions related to sparganosis have been reported in but a few cases.4-10)
Pericardial effusions are not uncommonly encountered in daily clinical practice as the cause of chest discomfort, dyspnea, and sudden hypotension. Diagnostic pericardiocentesis is frequently used to analyze the characteristics of pericardial fluid, and exudative or bloody pericardial effusions obtained during diagnostic pericardiocentesis should be studied meticulously using laboratory examinations and imaging modalities to define the cause of the pericardial effusion, which includes malignancies, uremia, large vessel trauma, or infections related to bacteria, viruses, or mycobacteria. Sometimes, surgical pericardiectomy and biopsy is essential to determine the cause of a pericardial effusion.11)12) In addition, a special diagnostic tool, such as an enzyme linked immunosorbent assay (ELISA) is necessary to diagnose sparganosis. Herein we report a rare form of human sparganosis that presented with a recurrent pericardiopleural effusion and peripheral eosinophilia without a subcutaneous mass or lump.
A 53-year-old woman sought evaluation in our cardiology outpatient clinic to for chest pain that persisted for 2 weeks in June 2005. The chest pain was described as "prickly," triggered mainly by deep inspiration, and unrelated to exercise. The patient also complained of a dry cough, arthralgias, generalized malaise, and a chilling sensation. She had taken medications for diabetes, hypertension, and hypothyroidism for 10 years. Her vital signs were as follows: blood pressure, 120/80 mmHg; heart rate, 130/minute; respiratory rate, 20/minute; and body temperature, 36.5℃. The initial laboratory test results were as follows: white blood cell (WBC) count, 10,800/µL with 26.9% eosinophils; erythrocyte sedimentation rate, 32 mm/hour; C-reactive protein level, 2.60 mg/dL; blood urea nitrogen, 15 mg/dL; serum creatinine, 0.9 mg/dL; total calcium, 9.6 mg/dL; phosphate, 4.0 mg/dL; alanine aminotransferase, 26 IU/L; aspartate aminotransferase, 16 IU/L; total bilirubin, 0.2 mg/dL; thyroid stimulating hormone, 0.75 µIU/mL; free thyroxine, 1.37 ng/dL; and total triiodothyronine, 63.80 ng/dL (n.b., the thyroid function tests were within normal limits because she had taken levothyroxine on a regular basis). Chest radiography showed mild cardiomegaly and a blunted left costophrenic angle without pulmonary congestion (Fig. 1A). Electrocardiography (ECG) showed atrial fibrillation with a rapid ventricular response, whereas normal sinus rhythm had been observed in a local clinic 2 weeks previously. Echocardiography demonstrated a pericardial effusion of maximal depth 12 mm, without hemodynamic significance (Fig. 1B and C). Chest computed tomography (CT) also showed a significant amount of pericardial effusion and a minimal amount of pleural effusion without nodular densities on the pleura or pericardium (Fig. 1D). The amounts of pericardial and pleural effusion were too small to perform a percutaneous needle aspiration to obtain a fluid sample. We decided on a 'watchful waiting' approach without specific treatment under a presumptive diagnosis of viral pericardiopleuritis. A follow-up chest radiography and echocardiography performed on the 6th hospital day showed near-resolution of the pericardiopleural effusion spontaneously. The patient was discharged 8 days after admission with symptomatic improvement.
One month after discharge, however, the patient returned to our outpatient clinic complaining of recurrent chest pain with a dry cough and dyspnea. Chest radiography revealed the reappearance of a pleural effusion (Fig. 2A). We performed a thoracentesis and pleural fluid analysis. The pleural fluid was yellow and contained 3,920/µL of red blood cells and 1,880/µL of WBCs (eosinophils accounted for 10% of all nucleated cells) (Fig. 2B). Protein and lactate dehydrogenase in the pleural fluid and serum were 6.89 g/dL and 389 IU/L, and 8.5 g/dL and 465 IU/L, respectively. Other pleural fluid results were as follows: specific gravity, 1.015; glucose, 171 mg/dL; and adenosine deaminase, 19 IU/L. The pleural fluid smear with gram stain, culture, and cytologic examination failed to detect Mycobacterium tuberculosis, ordinary bacteria, or malignant cells. As the day progressed, her cough and dyspnea improved with symptomatic treatment and the pericardiopleural effusion decreased in amount spontaneously on follow-up chest radiograph and echocardiogram performed on the 6th hospital day (Fig. 2C and D). The patient was discharged 8 days after the 2nd admission without any medications. The cause of the recurrent and spontaneously-absorbed exudative pericardiopleural effusion remained unclear.
A chest radiography obtained 10 days after the 2nd discharge from our institution showed an increased amount of pleural effusion again. Therefore, we planned video-assisted thoracic surgery to obtain pleura tissue and pericardium in an effort to determine the definitive cause of the recurrent pericardiopleural effusion. At the time of admission for surgery, we again reviewed her recent medical history meticulously, and found that she had eaten more than 30 raw frogs 3 months previously (at the beginning of May 2005) because her neighbors had advised her that raw frog flesh was good for thyroid disease.
Tuberculosis or malignancies, as the common causes of pericardial effusions, were ruled out by pleural fluid analysis (vide supra). We then entertained the cause of the pericardial effusion with peripheral eosinophilia to a parasitic infection, such as sparganosis, based on her history of ingesting the raw flesh of frogs. According to the general method for diagnosing human sparganosis, we rechecked the entire body for the presence of subcutaneous lumps or masses and searched for nodules in the pericardium or pleura by chest CT to establish the pathologic diagnosis. However, there was no evidence of masses or lumps. Therefore, we decided to perform an ELISA using sparganum antigen with a modified Voller's method.13) Briefly, microplates (Immuno Modules™; Nunc, Rochester, NY, USA) were coated with 5 µg/µL of sparganum antigens in carbonate buffer (pH 9.6) and probed with sera diluted to 1 : 20 in phosphate buffered saline (pH 7.4) containing 0.5% skim milk and 0.05% Tween-20. To detect antibodies bound to antigen, peroxidase-conjugated goat anti-human IgG (Cappel Co., Durham, NC, USA) was used diluted 1 : 1,000 in phosphate buffered saline (pH 7.4). The substrate for the color reaction was 0.1% O-phenylenediamine and the reaction was stopped by adding 50 µL of 4M H2SO4. Absorbances (Abs) were recorded at 492 nm using a micro-ELISA reader (Lab-systems, Helsnk: Finland). Anti-sparganum-specific IgG in serum by ELISA was significantly higher (Abs, mean±SD: 0.911±0.03) than the normal control level (maximum cut off Abs value: 0.2).
The patient was treated with 3 consecutive doses of praziquantel (75 mg/kg/day). Six weeks after treatment, the amount of pericardiopleural effusion had decreased markedly and the eosinophil count in the peripheral blood had returned to the normal range (387/µL; 5.1% of total WBCs).
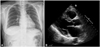
Four years after praziquantel treatment, the patient had a WBC count of 5,910/µL with 2% eosinophils (118/µL). The serum anti-sparganum-specific IgG level was reduced markedly to an Abs of 0.698 as compared with that of the initial diagnosis, but remained higher than the level of normal controls. A chest radiography and echocardiography showed no evidence of a pericardiopleural effusion (Fig. 3). She has not complained of any symptoms after treatment in over 4 years of follow-up.
Sparganosis is an infection caused by the plerocercoid larvae of various diphyllobothroid tapeworms, including the genus Spirometra. Humans are accidental secondary intermediate hosts and can be infected through three possible routes. The most common route of infection in humans is drinking water contaminated with cyclops containing procercoids, which penetrate the intestinal wall and then move to muscles or subcutaneous tissues. The second route is the ingestion of raw or partially cooked flesh of fish, frogs, snakes, and chickens containing plerocercoids, which may result in parasite migration through the intestinal wall. The third route is the poultice application of water infested with cyclops containing procerciods to open wounds or sore eyes, which is a common route in southeast Asia.3)14) Eating raw flesh of snakes or frogs is the most important and frequent route of infection in Far East Asia, especially in Japan and Korea. The reasons for consuming the raw flesh of these animals in Korea are to potentiate masculine activity, obtain special nutrition, cure some diseases, survive in combat, and because the flesh is liked.3) In this case, the patient ate raw frogs to treat her hypothyroidism.
About 500 cases of human sparganosis have been reported in the medical literature from diverse areas in the world. In Korea, most infections present as lumps in subcutaneous tissues or intermuscular fascia, which are commonly found in the abdominal wall, scrotum, lower extremities, and chest wall.3)14)15) Sometimes, infections involve the orbits, urinary tract, or body cavities, such as the abdomen or vertebral canal.16)17) However, only a few cases of pleural cavity involvement have been reported (10 cases of pleural sparganosis manifested with pleural effusion in Japan and 3 cases of pleural sparganosis in Korea, including 1 case of empyema and 1 case with a pleural nodule without a pleural effusion).4-10) However, the manifestation with pericardial effusion is extremely rare, and has not been previously reported, although one autopsy case was reported in Japan that the larvae was observed in the outer surface of the pericardium. Therefore, this is the first case of recurrent exudative pericardiopleural effusion in a presumed case of human sparganosis. Almost all patients with pleural effusions related to sparganosis have had a history of raw flesh ingestion and complained of non-specific symptoms, such as, malaise, dyspnea, and pleuritic chest pain.4-10) These previous cases had no subcutaneous lumps and showed lymphodominant exudative pleural effusions with eosinophilia and peripheral eosinophilia without significant leukocytosis, except for one case of an empyema. Our case had similar laboratory findings with the above cases.
Although the diagnosis of human sparganosis is usually established by pathologic examinations of worms or eggs, diagnosis is almost impossible in cases of pericardial or pleural effusions without a pleural nodule. In such cases, immunoserologic methods, such as ELISA, can be helpful to diagnose sparganosis18)19) based on positivity for parasite-specific IgG.6)10) Similarly, no subcutaneous lumps or pericardiopleural nodules were observed in our case and the amount of pericardial effusion was too small to obtain by pericardiocentesis initially, thus we could not help testing patient serum by ELISA to diagnose sparganosis as a possible cause of the recurrent pericardiopleural exudative effusion. According to the ELISA results of previous cases in our parasitology laboratory, ELISA using the sera of patients with sparganosis confirmed by pathologic examination showed the absorbance range from 0.45-1.0, whereas from 0.05-0.2 in the healthy controls. All patients with sparganosis showed cross-reactivity with Cysticercus cellulosae, with an abs ≥0.5 and there was no cross-reactivity with Clonorchis sinensis or Paragonimus westermani (unpublished internal data). In this patient, ELISA using sparganum and Cysticercus cellulosae antigen showed an abs of 0.911 and 0.57, respectively, which meant positive cross-reactivity in these 2 parasitologic diseases, but the ELISA using Clonorchis sinensis and Paragonimus westermani antigens showed negative results. These results are in agreement with our previous internal data. The diagnosis of our patient is more likely sparganosis than cysticercosis because she had eaten raw frogs rather than raw pork and no enhancing cystic lesions were observed in diagnostic imaging modalities. Clonorchiasis and paragonimiasis were ruled out based on negative responses to skin tests, repeated stool examinations, and ELISA results. Furthermore, normal thyroid function tests and the maintenance of thyroxine treatment eliminated the possibility of a pericardial effusion related to thyroid disease.
The principle treatment of human sparganosis is surgical removal; no medication has been proven to be an effective measure against sparganum.20) Even though surgical removal is the definitive treatment, it cannot be applied in some conditions, such as the pericardiopleural effusion associated with sparganosis without a pleural or pericardial nodule or lump. Some reports have advised that praziquantel is effective in pleural sparganosis. Tanaka et al.4) reported a case of pleural sparganosis successfully treated with praziquantel, and Ishii et al.5) treated a case of eosinophilic pleuritis due to sparganosis with 3 consecutive doses of praziquantel (75 mg/kg/day). After treatment, the anti-sparganum-specific antibody titer was decreased and the eosinophil count in peripheral blood returned to normal.5) The surgical method was not a treatment option in the present case because the patient did not have a subcutaneous lump or a pericardiopleural nodule. Therefore, we prescribed 3 consecutive doses of praziquantel (75 mg/kg/day), as in the aforementioned cases.4)6) Six weeks later, the serum anti-sparganum specific antibody titer in our patient was decreased and the eosinophil count in the peripheral blood had returned to normal.
We continue to follow this patient biannually for subcutaneous manifestations or pericardiopleural effusions related with sparganosis, but no additional findings have been detected in >4 years. We surmised that praziquantel converted the sparganosis from an active to a latent form of infection. Thus, we suggest that praziquantel is a therapeutic option for pericardial effusion related to sparganosis when the worms cannot be surgically removed. Although human sparganosis manifesting as a pericardial effusion is rare, it should be considered an important cause of eosinophilic pericardiopleural effusions.
Figures and Tables
Fig. 1
Images obtained at the time of first admission. A: chest radiograph showed mild cardiomegaly without pulmonary congestion and blunting of the left costrophrenic angle. B and C: echocardiogram demonstrated pericardial effusion with a maximal depth of 12 mm without hemodynamic significance. D: chest CT revealed pericardial effusion and a small amount of pleural effusion, but no other notable findings.
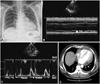
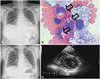
Fig. 2
Images obtained during the second admission. A: chest radiography showed increased amounts of pleural effusion. B: increased number of eosinophils was observed in the pleural fluid (black arrows) (wright stain, ×1,000). C and D: follow-up echocardiogram and chest radiograph before discharge showed reduced amounts of pleural effusion and the absence of a pericardial effusion.
References
1. Sun T. Parasitic Disorders: Pathology, Diagnosis, and Management. 1999. 2nd ed. Pennsylvania: Williams & Wilkins;285–290.
2. Mueller JF. The biology of Spirometra. J Parasitol. 1974. 60:3–14.
3. Cho SY, Bae JH, Seo BS. Some aspects of human sparganosis in Korea. Korean J Parasitol. 1975. 13:60–77.
4. Tanaka S, Maruyama H, Ishiwata K, et al. A case report of pleural sparganosis. Parasitol Int. 1997. 46:73–75.
5. Ishii H, Mukae H, Inoue Y, et al. A rare case of eosinophilic pleuritis due to sparganosis. Intern Med. 2001. 40:783–785.
6. Kamiya H, Shimizu H, Kobayashi N, Kudo K. A case of sparganosis with eosinophilic pleural effusion. Nihon Kokyuki Gakkai Zasshi. 2004. 42:1019–1023.
7. Shimada M, Matsumura K, Aoki Y, et al. Two cases of sparganosis having onsets of pleural effusion. Jpn J Parasitol. 1989. 38:145.
8. Yamasaki H, Araki K, Aoki T. Parasitic diseases examined during the past 16 years in the Department of Parasitology, Juntendo University School of Medicine. Juntendo Igaku. 1994. 40:262–279.
9. Kim DH, Yi IH, Youn HC, et al. Pleural sparganosis: a case report. Korean J Thorac Cardiovasc Surg. 2006. 39:502–504.
10. Koh TW, Lee JW, Sohn DS, et al. Empyema thoracis associated with sparganosis: a case report. Korean J Thorac Cardiovasc Surg. 1988. 21:761–765.
11. Ha JK, Hong TJ, Chun KJ, et al. Biochemical analysis of serum and pericardial fluid in patients with hemorrhagic pericardial effusion. Korean Circ J. 2003. 33:227–232.
12. Eo WK, Lee SK, Choi CJ, et al. Clinical studies on the etiology and clinical course of pericardial effusions. Korean Circ J. 1990. 20:211–219.
13. Voller A, Bidwell DE, Bartlett A. The Enzyme-Linked Immunosorbent Assay. 1994. London: Nuffield laboratories of comparative medicine;19–41.
14. Min DY. Cestode infections in Korea. Korean J Parasitol. 1990. 28:123–144.
15. Sim S, You JK, Lee IY, Im KI, Yong TS. A case of breast sparganosis. Korean J Parasitol. 2002. 40:187–189.
16. Kim HJ, Park SH, Yum DJ, et al. A case of sparganosis in the submandibular triangle. J Clin Otolaryngol Head Neck Surg. 2005. 16:330–333.
17. Cho KJ, Lee HS, Chi JG. Intramural sparganosis manifested as intestinal obstruction. J Korean Med Sci. 1987. 2:137–139.
18. Kim H, Kim SI, Cho SY. Serological diagnosis of human sparganosis by means of micro-ELISA. Korean J Parasitol. 1984. 22:222–228.
19. Yeo IS, Yong TS, Im K. Serodiagnosis of human sparganosis by a monoclonal antibody-based competition ELISA. Yonsei Med J. 1994. 35:43–48.
20. Sohn WM, Hong ST, Chai JY, Lee SH. Infectivity of the sparganum treated by praziquantel, gamma-irradiation and mechanical cutting. Korean J Parasitol. 1993. 31:135–139.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download