Abstract
QT prolongation is a serious adverse drug effect, which is associated with an increased risk of Torsade de pointes and sudden death. Many drugs, including both cardiac and non-cardiac drugs, have been reported to cause prolongation of QT interval. Although meperidine has not been considered proarrhythmic, we present a unique case of a 16-year-old boy without an underlying cardiac disease, who developed polymorphic ventricular tachycardia, ventricular fibrillation and QT prolongation after an intravenous meperidine injection. He had no mutation in long QT syndrome genes (KCNQ1, KCNH2, and SCN5A), but single nucleotide polymorphisms were reported, including H558R in SCNA5A and K897T in KCNH2.
Long QT syndrome (LQTS) is a disorder of myocardial repolarization characterized by a prolonged QT interval on an electrocardiogram (ECG), leading to Torsade de pointes (TdP) and ventricular fibrillation. QT prolongation may be congenital or acquired. In the acquired form of the syndrome, some medications have been confirmed to cause QT prolongation and TdP, which is the main reason for the withdrawal or restriction of the use of drugs that have already been marketed.1) Methadone, one of the opioid compounds, is well known to have a dose-response relationship with corrected QT (QTc) interval.2) Meperidine hydrochloride (Demerol®) is another synthetic opioid analgesic frequently prescribed for pain control. However, no adverse drug effects on arrhythmia have been reported. Here, we report a case of a patient with meperidine-induced QT prolongation and life-threatening polymorphic ventricular tachycardia.
A 16-year-old boy complained of chest tightness and dizziness immediately after an intravenous meperidine injection for pain control before a colonoscopy.
He had a history of generalized tonic-clonic seizure at the age of thirteen and had been taking valproate. His brain MRI was normal and his EEG showed generalized spike waves, implying generalized epilepsy. Otherwise, he was healthy and had no family history of syncope or sudden death. As he had complained of cramping abdominal pain with diarrhea 2-3 times per week for one year before this event, he was scheduled for a colonoscopy under the suspicion of inflammatory bowel disease. Initially, his vital signs were stable and his heart rate was 72 beats/min. After a meperidine injection for pain control before the colonoscopy, he complained of chest discomfort and lost consciousness. Three-lead ECG monitoring revealed polymorphic VT and ventricular fibrillation and cardiopulmonary resuscitation was performed (Fig. 1). The patient was successfully defibrillated with 300 Joules of direct current energy. After pulseless ventricular arrhythmia, his ECG showed a prolonged QTc interval, over 500 msec, and serum electrolytes were within the normal range. His QTc interval remained prolonged at 597 msec and his rhythm remained sinus bradycardia at 54 bpm the day after the event (Fig. 2). His QTc intervals normalized slowly over the following 3 days. He was transferred to our hospital 5 days after the event, showing a normal QTc interval of 424 msec and a heart rate of 67 bpm. To establish the repolarization abnormality, an epinephrine challenge test was performed.3) The QTc interval became prolonged up to 567 msec from the baseline (398 msec) 30 seconds after the epinephrine bolus injection (0.1 µg/kg), followed by continuous infusion (0.1 µg/kg/min) (Fig. 3A and B). The steady state QTc was 425 msec. Both the treadmill exercise test and 24-hour holter monitoring were normal, and there were no cardiac abnormalities seen on the echocardiogram. We performed a genetic analysis for 3 genes encoding essential channel subunits, including KCNQ1 (LQT1), KCNH2 (LTQ2), and SCN5A (LQT3). There was no related mutation, but 12 single nucleotide polymorphisms (SNPs), including H558R in SCNA5A and K897T in KCNH2, were reported in these genes. He also carried E1601E, D1819D, A29A, IVS9 and IVS10 variants in SCN5A, L564L; Y652Y and IVS13 variants in KCNH2; and A370A and P448R variants in KCNQ1. The patient had had a follow-up ECG, which had demonstrated normal QT intervals. There was no significant abnormality in the ST segment on the ECG during the follow-up period. After 1.5 years, he underwent an electrophysiological study. There was no QT prolongation after isoproterenol injection and arrhythmia was not induced by a pacing study. A treadmill exercise test, 24-hour holter monitoring, and an epinephrine test were repeated, showing neither abnormalities in rhythm nor QT prolongation (Fig. 3C). He experienced no chest tightness, dizziness, or syncope thereafter. He took a beta-blocker for 22 months after ventricular fibrillation and subsequently stopped taking medication. He was advised not to take meperidine or other QT-prolonging drugs.
The acquired form of LQTS is a potentially fatal medical condition, which can be exacerbated by a wide range of both cardiac and non-cardiac medications. Many of the drugs initially known to prolong QT intervals were Ia and III antiarrhythmics.4)5) However, many non-cardiac drugs have also been reported to cause QT prolongation, accounting for 2-3% of the total drug prescriptions in the UK and Italy.6) Drugs which are generally considered to confer a risk of TdP, are antibiotics (macrolides and quinolones), antidepressants (tricyclics and selective serotonin reuptake inhibitors), antipsychotics (haloperidol and phenothiazines), and antiemetics (ondansetron and prochlorperazine). Most of these agents exhibit direct electrophysiological effects on cardiac ion channels, usually by blocking rapidly activating delayed rectifier (repolarizing) potassium current (IKr). Many drugs, however, block multiple cardiac ion channels (IKr, IKs, and INa) leading to a more complex shift of action potential morphology. 1) These conditions cause a cardiac repolarization delay, inducing TdP. The HERG (human ether-à-go-go-related gene, alternative nomenclature KCNH2) is responsible for channels mediating IKr which plays an important role in ventricular repolarization. Pharmacological inhibition of native IKr and recombinant HERG channels is a shared feature of diverse drugs associated with TdP.7)
Meperidine or pethidine (commonly referred to as Demerol®) is an opioid analgesic drug. Katchman et al.8) described the ability that opioid compounds have in influencing the cardiac HERG → KCNH2 K+ current, IHERG. In opioids, L-α-acetylmethadol (LAAM) and methadone block IHERG in transfected cells at clinically relevant concentrations, which could explain the mechanisms for adverse cardiac effects observed in some patients receiving LAAM or methadone. Meperidine was less potent in IHERG inhibition than LAAM or methadone, but it is postulated that meperidine may act as a QT-prolonging drug by blocking IHERG in the study patient.
Multiple clinical risk factors for drug-induced TdP have been identified; female gender, hypokalemia, bradycardia, congestive heart failure, high drug concentrations, rapid intravenous infusion with a QT-prolonging drug, severe hypomagnesemia, subclinical LQTS, ion channel polymorphism, and base-line QT prolongation. Bradycardia is more likely to cause exaggerated QT prolongation in susceptible patients than in non-susceptible patients.9) In our patient, injection of intravenous meperidine induced bradycardia, which may have aggravated QT prolongation. The latter could explain the lengthening of cardiac repolarization observed in this patient receiving clinical doses of meperidine. Valproate, which he had been taking at the time, is not known to have an interaction with meperidine.
It has been recognized that drug-induced QT prolongation may depend on a genetic substrate.9-13) Several researchers have suggested that 5% to 10% of patients with drug-induced TdP have DNA variants in the coding regions of congenital long-QT disease genes and may have a subclinical form of the congenital disease.9)10)14) It has also been shown that not only rare mutations but also polymorphisms in LQTS genes could represent risk factors for drug-induced arrhythmia. In addition, some allelic variants were reported to influence QTc length even in healthy individuals, and may represent risk factors for arrhythmias or cardiac sudden death.15-17)
Our patient had no mutation associated with LQT1, LQT2, or LQT3, which account for most cases of LQTS. Nevertheless, he had 12 SNPs in the coding region and flanking region of SCN5A, KCNH2, and KCNQ1. P448R and A370A SNPs in KCNQ1 (LQT1), K897T, L564L; Y652Y SNPs in KCNH2 (LQT2), H558R, E1601E, D1819D, A29A; and IVS9 1141-3C>A SNPs in SCN5A (LQT3), were previously reported in drug-induced LQTS patients.11) Among them, H558R in SCN5A, a relatively common polymorphism present in 20-30% population, is associated with a prolonged QTc interval.14)16) K897T in KCNH2 is another common polymorphism and was reported to be associated with either short QT interval or long QT interval.18)19) H558R in SCN5A and K897T in KCNH2 have been confirmed to alter channel physiology and cardiac ion channel function, which may create a vulnerable substrate in the presence of appropriate triggers such as IKr blockers, precipitating life-threatening ventricular arrhythmias.16)20) Therefore, we speculated that meperidine might be the trigger of ventricular arrhythmia and QT prolongation in this patient, with underlying functional polymorphisms in the congenital LQTS genes.
In conclusion, there have been numerous non-arrhythmic drugs that can cause QT prolongation and TdP. However, meperidine has not previously been reported as a QT prolonging agent. This patient's genetic analysis revealed SNPs in congenital LQTS, including H558R in SCN5A and K897T in KCNH2. We reported a case of meperidine-induced QT prolongation in a patient with SNPs in congenital LQTS genes. Meperidine is commonly used for children and adults before painful procedures. Therefore we should be fully aware of this fact and continue to carefully monitor proarrhythmic effects attributable to meperidine.
Figures and Tables
Fig. 2
Electrocardiogram the day after ventricular fibrillation developed. QTc interval is 591 msec.
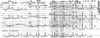
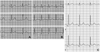
Fig. 3
Epinephrine test results. Six days after cardiopulmonary resuscitation, baseline electrocardiogram reveals normal QTc interval (A: QTc 431 msec), but 30 seconds after epinephrine bolus injection (0.1 µg/kg), followed by continuous infusion (0.1 µg/kg/min), QTc interval became prolonged up to 587 msec (B). Two years after cardiopulmonary resuscitation, epinephrine test shows normal (C: QTc 418 msec).
References
1. Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004. 350:1013–1022.
2. Wedam EF, Bigelow GE, Johnson RE, Nuzzo PA, Haigney MC. QT-interval effects of methadone, levomethadyl, and buprenorphine in a randomized trial. Arch Intern Med. 2007. 167:2469–2475.
3. Shimizu W, Noda T, Takaki H, et al. Diagnostic value of epinephrine test for genotyping LQT1, LQT2, and LQT3 forms of congenital long QT syndrome. Heart Rhythm. 2004. 1:276–283.
4. Kim SM, Kim DS, Kim DI, et al. Quinidine-induced QTc interval prolongation and gender differences in healthy Korean subjects. Korean Circ J. 2007. 37:559–566.
5. Velebit V, Podrid P, Lown B, Cohen BH, Graboys TB. Aggravation and provocation of ventricular arrhythmias by antiarrhythmic drugs. Circulation. 1982. 65:886–894.
6. De Ponti F, Poluzzi E, Montanaro N, Ferguson J. QTc and psychotropic drugs. Lancet. 2000. 356:75–76.
7. Hoffmann P, Warner B. Are hERG channel inhibition and QT interval prolongation all there is in drug-induced torsadogenesis? A review of emerging trends. J Pharmacol Toxicol Methods. 2006. 53:87–105.
8. Katchman AN, McGroary KA, Kilborn MJ, et al. Influence of opioid agonists on cardiac human ether-a-go-go-related gene K(+) currents. J Pharmacol Exp Ther. 2002. 303:688–694.
9. Donger C, Denjoy I, Berthet M, et al. KVLQT1 C-terminal missense mutation causes a forme fruste long-QT syndrome. Circulation. 1997. 96:2778–2781.
10. Napolitano C, Schwartz PJ, Brown AM, et al. Evidence for a cardiac ion channel mutation underlying drug-induced QT prolongation and life-threatening arrhythmias. J Cardiovasc Electrophysiol. 2000. 11:691–696.
11. Paulussen AD, Gilissen RA, Armstrong M, et al. Genetic variations of KCNQ1, KCNH2, SCN5A, KCNE1, and KCNE2 in drug-induced long QT syndrome patients. J Mol Med. 2004. 82:182–188.
12. Sesti F, Abbott GW, Wei J, et al. A common polymorphism associated with antibiotic-induced cardiac arrhythmia. Proc Natl Acad Sci U S A. 2000. 97:10613–10618.
13. Hyun DW, Han SW, Jo YK, et al. Moleculogenetic characteristics of the patient with long QT syndrome in Korean. Korean Circ J. 2004. 34:813–819.
14. Yang P, Kanki H, Drolet B, et al. Allelic variants in long-QT disease genes in patients with drug-associated torsades de pointes. Circulation. 2002. 105:1943–1948.
15. Albert CM, MacRae CA, Chasman DI, et al. Common variants in cardiac ion channel genes are associated with sudden cardiac death. Circ Arrhythm Electrophysiol. 2010. 3:222–229.
16. Gouas L, Nicaud V, Berthet M, et al. Association of KCNQ1, KCNE1, KCNH2 and SCN5A polymorphisms with QTc interval length in a healthy population. Eur J Hum Genet. 2005. 13:1213–1222.
17. Gouas L, Nicaud V, Chaouch S, et al. Confirmation of associations between ion channel gene SNPs and QTc interval duration in healthy subjects. Eur J Hum Genet. 2007. 15:974–979.
18. Pietila E, Fodstad H, Niskasaari E, et al. Association between HERG K897T polymorphism and QT interval in middle-aged Finnish women. J Am Coll Cardiol. 2002. 40:511–514.
19. Bezzina CR, Verkerk AO, Busjahn A, et al. A common polymorphism in KCNH2 (HERG) hastens cardiac repolarization. Cardiovasc Res. 2003. 59:27–36.
20. Crotti L, Lundquist AL, Insolia R, et al. KCNH2-K897T is a genetic modifier of latent congenital long-QT syndrome. Circulation. 2005. 112:1251–1258.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download