Abstract
Pericardiectomy is the standard treatment in patients with chronic constrictive pericarditis who have persistent symptoms. However, myocardial atrophy with prolonged pericardial constriction and abrupt increase in venous return can lead to heart failure with volume overload after pericardial decompression, especially in the right ventricle (RV). We experienced a 44 year old male patient who developed transient RV failure after pericardiectomy for constrictive pericarditis. Echocardiography revealed a markedly dilated RV with decreased peak systolic velocity of the tricuspid annulus, suggesting severe RV dysfunction. After treatment with inotropics and diuretics, a follow-up echocardiography revealed an improved systolic function with decreased RV chamber size. This case demonstrates the importance of volume overload and RV dysfunction in patients with constrictive pericarditis undergoing pericardiectomy.
Constrictive pericarditis is a condition where loss of pericardial elasticity causes restriction on diastolic filling of the heart.1) Surgery is the accepted standard treatment in patients with chronic constrictive pericarditis who have persistent symptoms. However, myocardial atrophy with prolonged constriction and rapid increase in venous return can lead to heart failure with volume overload after pericardial decompression, especially in the right ventricle (RV). Doppler echocardiography is an effective method for monitoring such hemodynamic changes after pericardiectomy.2) We report an interesting case of a 44-year-old male patient who developed transient RV failure after pericardiectomy for constrictive pericarditis.
A 44 year old man was referred to our hospital because of progressive dyspnea, edema in both legs, and uncontrolled pleural effusion with uncertain etiologies for two months. The patient underwent thoracentesis with a pleural biopsy at a private clinic, where he initially presented, but failed to reveal the cause of pleural effusion. Despite the absence of confirmative diagnosis, empirical anti-tuberculosis medication was initiated, suspecting tuberculosis pleurisy. The patient had no particular medical history except for mental retardation, and denied any alcohol or tobacco use.
Physical examination revealed decreased breath sound at the right lower lung field and mild pitting edema on both pretibial areas. An electrocardiography showed a normal sinus rhythm at a rate of 93 beats/min, and a chest X-ray revealed cardiomegaly with bilateral pleural effusion. A two-dimensional echocardiography demonstrated pericardial thickening with adhesion, septal bouncing motion, and dilated inferior vena cava with plethora. Doppler showed respiratory variation of mitral inflow early velocity (42%) and expiratory diastolic flow reversal of the hepatic vein, suggesting constrictive pericarditis. It also revealed preserved early diastolic velocity (E' 9.0 cm/s) of the mitral annulus and normal peak systolic velocity (S' 15.5 cm/s) of the tricuspid annulus (Figs. 1A and 2A). The RV end-diastolic volume, RV end-systolic volume and RV ejection fraction were 34.6 mL, 20.3 mL and 41% (Fig. 3A and B). These findings were compatible with RV systolic dysfunction. With a diagnosis of constrictive pericarditis probably due to tuberculosis origin, anti-tuberculosis medication and diuretics were maintained.
Despite more than six months of medical therapy, there was no significant improvement in chest X-ray and repeated drainage was performed for medically refractory pleural effusion. Therefore, the patient underwent pericardiectomy via left anterior thoracotomy. On the third postoperative day, a chest X-ray revealed increased right pleural effusion. An echocardiography showed improved constrictive physiology with normal global left ventricular (LV) systolic function and decreased E' (4.8 cm/s). However, markedly dilated RV with severe global hypokinesia was observed (tricuspid annulus S' 6.0 cm/s) (Figs. 1B and 2B). The RV end-diastolic volume and RV end-systolic volume markedly increased to 85.6 mL and 60.5 mL with further decreased RV ejection fraction (29%) (Fig. 3C and D). A chest computed tomography scan revealed no evidence of pulmonary thromboembolism. We promptly started dobutamine infusion and added diuretics for RV dysfunction with volume overload. After four days of medical therapy and salt restriction, follow up echocardiography showed a decreased RV chamber size with improved RV systolic function (tricuspid annulus S' 7.6 cm/s). The patient's symptoms were also much improved and he was discharged from the hospital with low dose oral diuretics.
About seven months later, a chest X-ray showed no pleural effusion and an echocardiography demonstrated more improved RV systolic function (tricuspid annulus S' 8.0 cm/s) without evidence of previous constrictive physiology (Figs. 1C and 2C). The RV end-diastolic volume and end-systolic volume were normalized to 59.7 mL and 26.6 mL with improved RV ejection fraction (55%) (Fig. 3E and F). He is currently being followed up in the outpatient department without any medication.
Echocardiography is a helpful and essential diagnostic tool in patients with constrictive pericarditis. The two-dimensional echocardiographic findings of increased pericardial thickness, abnormal septal motion, atrial enlargement, and dilation with diminished collapse of the inferior vena cava and hepatic veins (plethora) are supportive of the diagnosis.3) Tissue Doppler shows a prominent or preserved E', since the longitudinal motion of the myocardium is enhanced because of constricted radial motion.4) In this case, preserved E' on preoperative echocardiography was clearly decreased on follow up echocardiography following pericardiectomy. A diminution of E' on tissue Doppler echocardiography, so to speak, can be used as an indicator of improved constrictive physiology after treatment.
Previous studies have described RV failure in the absence of a specific cause. Dilation of the RV related to pericardial disease has been reported after pericardiectomy,5) congenital absence of the pericardium,6) and following therapeutic pericardiocentesis for cardiac tamponade.7) In this case, we have described the favorable outcome of a transient RV dysfunction following pericardiectomy due to constrictive pericarditis.
Several possible mechanisms can be considered for the pathophysiology of RV dysfunction after pericardiectomy in patients with constrictive pericarditis. For example, anatomic studies in cases of constrictive pericarditis have demonstrated the presence of myocardial atrophy.8) Myocardial atrophy, which is attributed to ventricular immobilization from prolonged constriction, can lead to dilation of cardiac chambers with low cardiac output.
In patients with constrictive pericarditis, however, the echocardiographic parameter of RV systolic function (tricuspid annulus S') may be exaggerated due to enhanced longitudinal motion of myocardium, just like in the case with prominent E' mentioned earlier. So, the disuse myocardial atrophy of RV can be masked on preoperative echocardiography, as we can see in the present case.
Another explanation involves a muscular injury possibly related to impaired coronary flow by a rapid increase in RV wall tension.9) Frequently, the RV visibly expands during pericardiectomy.10) Pericardial decompressions may cause a sudden increase in venous return resulting from an abrupt decrease in right atrial pressure, and the increased venous return will mainly affect the RV, which is one of the most distensible cardiac chambers.
Massive pulmonary thromboembolism may mimic the echocardiographic findings of the dilated and severe hypocontractile RV. In the present case, the transient RV dysfunction was not due to pulmonary thromboembolism. Computed tomography or a ventilation-perfusion scan will help to rule out this disease entity.
This case demonstrates the importance of special attention for volume overload and RV dysfunction in patients with constrictive pericarditis undergoing pericardiectomy. Echocardiography can reveal a dramatic change in hemodynamic parameters, reflecting an eventful clinical course for patients after pericardiectomy. Even with a preserved LV systolic function, inotropics support may be necessary in patients with signs of RV failure. Volume control with diuretics and salt restriction will be helpful in this condition until the heart function recovers.
Figures and Tables
Fig. 1
Left ventricular tissue Doppler image shows preserved early diastolic mitral annular velocity (E') on preoperative echocardiography (A), decreased E' on postoperative (B) and 7 months follow up echocardiography (C).

Fig. 2
Right ventricular tissue Doppler image shows normal tricuspid annular velocity (S') on preoperative echocardiography (A), reduced S' on postoperative echocardiography (B), and slightly increased S' on echocardiography of 7 months follow up (C).

Fig. 3
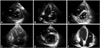
Two-dimensional echocardiography of the parasternal short-axis view (A, C and E) and apical 4-chamber view (B, D and F). The echocardiography showed pericardial thickening and relatively small sized RV (end-diastolic volume: 34.6 mL) at admission (A and B). Postoperative echocardiography revealed markedly dilated RV (end-diastolic volume: 85.6 mL) with D-shaped LV (C and D). The RV size was decreased (end-diastolic volume: 59.7 mL) on echocardiography after 7 months (E and F). RV: right ventricle, LV: left ventricle.
References
1. Nishimura RA. Constrictive pericarditis in the modern era: a diagnostic dilemma. Heart. 2001. 86:619–623.
2. Ha CB, Huh JY, Shin YW, Shin YK. Doppler flow patterns of constrictive pericarditis. Korean Circ J. 1989. 19:47–54.
3. Talreja DR, Edwards WD, Danielson GK, et al. Constrictive pericarditis in 26 patients with histologically normal pericardial thickness. Circulation. 2003. 108:1852–1857.
4. Ha JW, Oh JK, Ling LH, Nishimura RA, Seward JB, Tajik AJ. Annulus paradoxus: transmitral flow velocity to mitral annular velocity ratio is inversely proportional to pulmonary capillary wedge pressure in patients with constrictive pericarditis. Circulation. 2001. 104:976–978.
5. Viola A. The influence of pericardiectomy on the hemodynamics of chronic constrictive pericarditis. Circulation. 1973. 48:1038–1042.
6. Payvandi M, Kerber R. Echocardiography in congenital and acquired absence of the pericardium: an echocardiographic mimic of right ventricular volume overload. Circulation. 1976. 53:86–92.
7. Anguera I, Pare C, Perez-Villa F. Severe right ventricular dysfunction following pericardiocentesis for cardiac tamponade. Int J Cardiol. 1997. 59:212–214.
8. Dines DE, Edwards JE, Burchell HB. Myocardial atrophy in constrictive pericarditis. Proc Staff Meet Mayo Clin. 1958. 33:93–99.
9. Geffroy A, Beloeil H, Bouvier E, Chaumeil A, Albaladejo P, Marty J. Prolonged right ventricular failure after relief of cardiac tamponade. Can J Anaesth. 2004. 51:482–485.
10. Bashi V, John S, Ravikumar E, Jairaj P, Shyamsunder K, Krishnaswami S. Early and late results of pericardiectomy in 118 cases of constrictive pericarditis. Thorax. 1988. 43:637–641.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download