Abstract
Double-chambered right ventricle (DCRV) is a rare congenital heart disorder in which the right ventricle is divided by an anomalous muscle bundle into a high pressure inlet portion and a low pressure outlet portion. We report a case of isolated DCRV without symptoms in adulthood, diagnosed through echocardiography, cardiac catheterization and cardiac magnetic resonance imaging.
Double-chambered right ventricle (DCRV) is a rare congenital heart disease characterized by the division of the right ventricular cavity into two chambers by anomalous muscle bundles.1) Typically, DCRV is diagnosed at childhood or adolescence, and most DCRV patients have associated congenital anomalies, such as ventricular septal defect (VSD), pulmonary stenosis, and subaortic stenosis.2) Here, we report a case of an isolated adult DCRV patient who had no symptoms.
A 26-year-old man was referred to the hospital for the evaluation of his abnormal cardiac murmur found during a routine health examination program. The patient had no cardiac symptoms, such as chest pain or dyspnea. During the physical examination, a prominent grade 5/6 systolic ejection murmur was heard on the left parasternal border. His blood pressure was 110/72 mmHg, and his heart rate was 66/min. In addition to mild cardiomegaly on the chest radiography, the electrocardiogram showed increased amplitude of the R wave on V1 and inverted T waves on V1-V3, suggesting right ventricular overload. The two-dimensional (2-D) transthoracic echocardiogram on the parasternal short axis view showed a marked muscle band protruding from the right ventricular free wall to the interventricular septum (Fig. 1A). In addition, right atrial enlargement, right ventricular hypertrophy and dilation, and moderate tricuspid regurgitation were found. A turbulent Doppler color flow jet with a mosaic pattern was seen through the stenotic mid-right ventricle on the parasternal short axis view (Fig. 1B). Continuous wave Doppler revealed tricuspid regurgitation between the right atrium and right ventricle with a flow acceleration of 7.0 m/s, corresponding to a pressure gradient of 196 mmHg calculated using the simplified Bernoulli equation (Δp=4ν2) (Fig. 1C).3) Transesophageal echocardiography demonstrated an anomalous muscle bundle dividing the right ventricle into two parts (Fig. 2). There was no shunt flow between the right and left parts of the heart. In addition, cardiac magnetic resonance imaging (MRI) revealed that hypertrophied muscle bundles transected the right ventricle from the free wall to the ventricular septum, resulting in the division of the right ventricle into two chambers (Fig. 3). Coronary angiography revealed normal coronary arteries; however the right ventriculogram demonstrated severe right ventricular muscle bundle hypertrophy, separating the right ventricle into the inflow and outflow chambers (Fig. 4). Cardiac catheterization was performed, and the pull-back pressure from right heart catheterization was recorded. The systolic pressure of the right ventricular inlet and the outlet pressure were 135 and 25 mmHg respectively, while the pressure gradient of the right ventricle was 110 mmHg (Fig. 5). There was no pressure gradient between the right ventricle outlet tract and the main pulmonary artery. Based on these findings, the patient was referred to a thoracic and cardiovascular surgery clinic for surgical correction, but the patient refused operative correction and was thus discharged, with local follow up.
DCRV is a rare form of congenital heart disease in which the right ventricle is divided into a proximal high pressure chamber and a distal low pressure chamber. There are several subtypes of divided right ventricle. A simple classification of the associated pathology was proposed by Galiuto, who divided DCRV into two distinct types of intracavitary obstruction.4) Type 1 DCRV is characterized by an anomalous muscle bundle crossing the right ventricular cavity, recognized as the cause of the intraventricular obstruction. In type 2 DCRV however, no anomalous muscle bundle is found. The obstruction in this case is caused by marked parietal and septal muscle hypertrophy. The intraventricular pressure gradient of type 1 DCRV is greater than that of type 2 DCRV, whereas VSD was found to be highly associated with type 2 DCRV.4) The patient in the case presented herein falls under the category of type 1 DCRV.
Associated cardiac anomalies are common in DCRV. The most common associated cardiac anomaly is VSD, present in 63-77% of the cases, while other reported associated anomalies include valvar pulmonary stenosis, atrial septal defect, aortic valve regurgitation, tricuspid valve regurgitation, persistent left superior vena cava, ruptured sinus of valsalva aneurysm, tetralogy of Fallot, complete or corrected transposition of the great arteries, and Ebstein anomaly.1)2) Thus, it is necessary to look for associated congenital anomalies when DCRV is diagnosed. Most cases of DCRV are diagnosed and treated during childhood, but the right ventricular outflow tract is not always a routine part of the adult echocardiographic examination. It can be difficult to obtain an image owing to the proximity of the right ventricular outflow tract to the transducer.5) If an image is available, the subcostal plane has the most diagnostic value.6) In older patients however, the parasternal short axis at the level of the aortic valve is found to be very useful,7) and in these cases, of superior value to the subcostal plane. Imaging with transesophageal echocardiography from the esophageal junction provides an excellent visualization of the right ventricle and coronary sinus because the gastroesophageal junction is close to the right atrium, coronary sinus, and right ventricle. Therefore, whether transesophageal echocardiography is helpful in reducing misdiagnosis prior to operation for VSD associated with DCRV remains to be seen.8) Finally, cardiac MRI has enabled three-dimensional visualization not only of the cardiac chambers and great vessels but also of the coronary artery system, with excellent spatial resolution. This may prove to be the most effective noninvasive means of imaging DCRV.9)
The nature of DCRV entails a tendency towards progressive obstruction by the muscle bundles, presumably secondary to both muscle hypertrophy and endocardial fibrosis. Patients can develop severe and varied symptoms, such as easy exhaustion, shortness of breath, recurrent chest infection, and in younger patients, retardation of growth. Therefore, treatment of most DCRV cases is surgical which often constitutes a part of the corrective procedure. Generally, the surgical procedures consist of the resection of the anomalous muscular bundle and correction of the associated cardiac anomalies. The period for surgical repair usually depends on the associated cardiac anomalies. In the absence of a significant coexisting defect, observation is possible as long as the intracavitary systolic gradient is not greater than 40 mmHg and the obstruction is not progressive.10) The long term prognosis for patients after the intracardiac repair of DCRV is excellent, but postoperatively, three-quarters of the patients were shown to have had complete or incomplete right bundle branch block in some studies.2)11) There are some cases, however, where antiarrhythmic agents or catheter ablation are required to treat ventricular tachycardia, or the insertion of a permanent pacemaker is required to treat the atrioventricular block during the postoperative period.8)11)12)
Most cases of DCRV are diagnosed during childhood and are commonly associated with VSD. However, if DCRV without an associated anomaly shows a tendency towards a slowly progressive obstruction, it is difficult to diagnose DCRV without symptoms. In our case where the patient had no prior medical history or symptoms, DCRV was accidentally diagnosed through the auscultation of a cardiac murmur during a routine pre-employment medical exam. Furthermore, in other studies concerning DCRV, patients were incidentally diagnosed during a non-cardiac surgery pre-operative examination, similar to what transpired in our case.9)13) 2-D echocardiography demonstrated right ventricular pressure overload and moderate tricuspid regurgitation, but there was no pulmonary stenosis or right ventricular outflow tract (RVOT) obstruction. The pressure gradient of the right ventricle added to the right atrial pressure is equal to the right ventricular systolic pressure. Estimates of right ventricular pressure can be helpful in the diagnosis of a variety of disorders, including pulmonary hypertension, RVOT obstruction and pulmonary stenosis.14) However, in this case, abnormal muscular obstruction in the right ventricle caused a pressure gradient. Therefore when evaluating a patient with elevated right ventricular systolic pressure, it is better to view the entire right heart complex before considering pulmonary hypertension, especially if there is no RVOT obstruction or pulmonary stenosis present.
Figures and Tables
Fig. 1
Double-chambered right ventricle demonstrated by transthrorasic echocardiography. A: two-dimensional echocardiography showing a stenotic mid-right ventricle with an anomalous muscle bundle (arrow). B: turbulent Doppler color flow with mosaic pattern. C: continuous-wave Doppler showing tricuspid regurgitation between the right atrium and right ventricle and indicating the flow acceleration and pressure gradient. RV: right ventricle, LV: left ventricle, RA: right atrium.
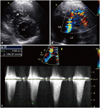
Fig. 2
Transesophageal echocardiography showing anomalous muscular bundles (arrow) dividing the right ventricle into two parts. RV: right ventricle, LV: left ventricle, RA: right atrium, LA: right atrium.
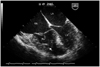
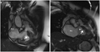
Fig. 3
Two-dimensional cine MRI showing anomalous muscle bundle (arrow). RV: right ventricle, RA: right atrium.
References
1. Cil E, Saraclar M, Özkutlu S, et al. Double-chambered right ventricle: experience with 52 cases. Int J Cardiol. 1995. 50:19–29.
2. Kim CJ, Chai IH, Koh KK, et al. Double chambered right ventricle in adult and adolescence. Korean Circ J. 1990. 20:248–255.
3. Nishimura RA, Miller FA Jr, Callahan MJ, Benassi RC, Seward JB, Tajik AJ. Doppler echocardiography: theory, instrumentation, technique, and application. Mayo Clin Proc. 1985. 60:321–343.
4. Galiuto L, O'Leary PW, Seward JB. Double-chambered right ventricle: echocardiographic feature. J Am Soc Echocardiogr. 1996. 9:300–305.
5. Choi YJ, Park SW. Characteristics of double-chambered right ventricle in adult patients. Korean J Intern Med. 2010. 25:147–153.
6. Hoffman P, Wojcik AW, Rozanski J, et al. The role of echocardiography in diagnosing double chambered right ventricle in adult. Heart. 2004. 90:789–793.
7. Galal O, Al-Halees Z, Solymar L, et al. Double-chambered right ventricle in 73 patients: spectrum of the disease and surgical results of transartial repair. Can J Cardiol. 2000. 16:167–174.
8. Chang RY, Kou CH, Rim RS, Chou YS, Tsai CH. Transesophageal echocardiographic image of double-chambered right ventricle. J Am Soc Echocardiogr. 1996. 9:347–352.
9. Sato Y, Matsumoto N, Matsuo S, et al. Double-chambered right ventricle: depiction at three-dimensional whole heart magnetic resonance imaging. Int J Cardiol. 2007. 119:e14–e16.
10. McElhinney DB, Chatterjee KM, Reddy VM. Double-chambered right ventricle presenting in adulthood. Ann Thorac Surg. 2000. 70:124–127.
11. Kim J, Park TI, An SG, et al. Electrical storm late after surgery for a double-chambered right ventricle, aortic regurgitation and a ventricular septal defect: a case of successful catheter ablation. Korean Circ J. 2008. 38:60–65.
12. Hachiro Y, Takagi N, Koyanagi T, Morikawa M, Abe T. Repair of double-chambered right ventricle: surgical results and long-term follow-up. Ann Thorac Surg. 2001. 72:1520–1522.
13. Yang SM, Chung WJ, Oh KJ, Kim MJ, Kim MK, Ahn TH. Two cases of double-chambered right ventricle without other congenital cardiac anomalies. J Korean Soc Echocardiogr. 2005. 13:37–41.
14. Vieillard-Baron A. Assessment of right ventricular function. Curr Opin Crit Care. 2009. 15:254–260.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download