Abstract
Background and Objectives
The aim of this study was to identify the association of pregnancy-induced hypertension (PIH) or gestational diabetes mellitus (GDM) with the development of venous thromboembolism (VTE).
Subjects and Methods
This was a retrospective study of 57,009 pregnancies during 2002-2008 at Cheil General Hospital, Kwandong University. The diagnosis of VTE {deep vein thrombosis or pulmonary embolism (PE)} was based on clot visualization via ultrasound or computed tomography.
Results
In total, 27 cases (PE, 20 cases) were detected. The incidence of VTE was 0.47 per 1,000 pregnancies. To determine risk factors associated with pregnancy-induced VTE, univariate analysis using a chi-square test was performed. Cesarean (C)-section, multiple pregnancy, PIH, placenta previa, and assisted reproduction technique (ART) were statistically significant compared to the controls (all, p=0.000). However, age, premature rupture of membrane, and GDM were not statistically related to VTE. Logistic regression analysis was used to calculate the odds ratios for the risk factors. Placenta previa showed a 12.6-fold higher risk, while PIH had a 9.8-fold higher risk for the occurrence of VTE. C-section and ART procedures increased the risk of VTE by 4.2 times compared to that of the controls.
Venous thromboembolism (VTE) is one of the leading causes of maternal morbidity and mortality in pregnancy.1) The incidence of VTE is estimated to be 0.76 to 1.72 per 1,000 pregnancies, which is four times greater than the risk in the non-pregnant population.2) Pregnancy itself induces a prothrombotic state with an increase in coagulation factors, a decrease in natural anticoagulants such as the coagulation inhibitor protein S, and impairment of fibrinolysis, which is probably mediated by an increase in plasminogen activator inhibitor.3) These procoagulant changes are important for minimizing blood loss during delivery. Pregnancy is also marked by the presence of two other components of Virchow's triad, venous stasis and endothelial injury. These homeostatic changes cause an increased risk for VTE.3)
A hypercoagulable state during pregnancy is known to be the most important factor for increasing the risk of VTE. In addition to inherited thrombophilia or the antiphospholipid syndrome, the assisted reproduction technique (ART), cesarean (C)-section, age older than 35 years, obesity, multiple pregnancies, placenta previa, pregnancy-induced hypertension (PIH), and gestational diabetes mellitus (GDM) are also known risk factors.1)4-8) Particularly among pregnancy-related diseases, PIH and GDM are common during pregnancy, with incidences of 6% to 8%, and 2.2% to 8.8%, respectively, and are known as VTE risk factors.9)10) However, recent research has questioned the relevance of PIH or diabetes mellitus (DM) to VTE.11)12) Therefore, this study was undertaken to identify the risk factors for the development of VTE in pregnancy, and to determine the contributions of PIH and GDM.
This was a retrospective study of 27 VTE patients out of 57,009 pregnancies at Cheil General Hospital from January 2002 to December 2008. Case controls were 56,982 patients who did not have VTE during or within four weeks after pregnancy, within the same time period.
The general characteristics of patients and known risk factors for VTE, including premature rupture of membrane (PROM), GDM, PIH, C-section, and ART were obtained through chart review. The study was approved by the Institutional Review Board of Cheil General Hospital, and authorization for the use of data retrieved from medical records for research purposes was also obtained. Only patients with manifestations of proximal deep vein thrombosis (DVT) and pulmonary embolism (PE) were considered to have VTE and were studied. The diagnosis of DVT was objectively confirmed by an intraluminal filling defect and noncompression viewed by color Doppler ultrasonography. PE was confirmed by observation of an intraluminal filling defect on computed tomography pulmonary angiography.
PIH includes gestational hypertension, preeclampsia/eclampsia, and preeclampsia superimposed on chronic hypertension. To diagnose GDM, a 50 g oral glucose tolerance (OGT) test was conducted at 24-28 weeks of pregnancy. When the glucose level in a 50 g OGT test was greater than 140 mg/dL, a 100 g OGT test was performed. According to the Carpenter-Coustan criteria,13) GDM was diagnosed when patient glucose levels exceeded two or more of the following thresholds: fasting glucose, 95 mg/dL; one hour glucose, 180 mg/dL; two hour, 155 mg/dL; or three hour, 140 mg/dL.
Statistical Package for the Social Sciences (SPSS) 12.0 for Windows (SPSS, Chicago, IL, USA) was used for statistical analysis. Results were expressed as the mean±SD. Risk factors for VTE were analyzed using a Chi-square test and linear logistic regression models, and are presented as risk ratios with 95% confidence interval (CI). Statistical significance was considered to be p<0.05.
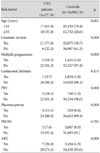
There were 27 VTE patients identified out of 57,009 deliveries, for a frequency of 0.47 VTE patients/1,000 deliveries, which is similar to the results of other studies. The mean patient age was 31.8±3.5 years (17-53 years) at the time of delivery. In this study, the frequency of PE was 0.035%, higher than the 0.012% for DVT. The rate for C-section delivery was 38.8%, for multiple pregnancies was 2.6%, for GDM was 1.9%, for PIH was 1.3%, for placenta previa was 0.6%, and for PROM was 8.9%. ART was used in 4.3% of patients (Table 1).
To identify the risk factors associated with pregnancy-induced VTE, univariate analysis using a Chi-square test was performed. As variables, PIH and GDM, in addition to traditional risk factors for VTE, such as age, C-section, multiple pregnancy, placenta previa, and ART were included. C-section (p=0.000), multiple pregnancy (p=0.000), PIH (p=0.000), placenta previa (p=0.000), and ART (p=0.000) were statistically significant compared to the controls. However, age (p=0.061), PROM (p=0.782), and GDM (p=0.475), which are thought to be risk factors for VTE, were not statistically associated with VTE (Table 2). Using logistic regression analysis, the odds ratios of risk factors were calculated. Placenta previa had a 12.6-fold higher risk, and PIH had a 9.8-fold higher risk for the occurrence of VTE. C-section and ART procedures increased the risk for VTE 4.2-fold compared to the controls. Multiple gestations, which showed a close correlation by univariate analysis, demonstrated no significant association (p=0.149) (Table 3) (Fig. 1). Age, GDM, and placenta abruptio did not increase the risk for VTE during or after pregnancy.
In this study, we found that PIH was closely correlated with pregnancy-induced VTE. Another new finding was that placenta previa contributed to the development of VTE, which has not been seen in other studies. Moreover, we found that there was no association of VTE with patient age or GDM, which are usually assumed to be VTE risk factors in pregnancy.
Because VTE during pregnancy or after delivery may be fatal to both mother and baby, understanding the risk factors and providing early thromboprophylaxis can help prevent VTE development.14) More than 50% of VTE occurs in the first two trimesters, and use of early thromboprophylaxis has been emphasized during pregnancy of patients at high risk for VTE. One of the most important risk factors for VTE in pregnancy is a history of thrombosis.11)15-17) In addition to thrombophilia, many studies have reported that C-section, PIH, multiparity, multiple gestation, GDM, and age (age >35) are risk factors for VTE in pregnancy.7)18)19) In a large Swedish study, C-section increased the risk of postpartum thrombosis by 5-fold,7) and this result was confirmed in another study.19)
Preeclampsia is known as a significant risk factor for VTE. Lindqvist et al.7) found no association of preeclampsia with antepartum VTE, but preeclampsia increased the risk of postpartum VTE by 3-fold. However, Ros et al.8) reported that preeclampsia was associated with both antepartum and postpartum VTE. In severe preeclampsia, the risk for VTE was elevated 4.8-fold. In another study determining the association of hypertensive pregnancy with cardiovascular (CV) and thromboembolic events, gestational hypertension, mild preeclampsia, and severe preeclampsia increased the risk for VTE by 2.8-, 2.2-, and 3.3-fold, respectively.20) A number of mechanisms may explain the observed association between VTE and CV disease. Adverse physiological changes developing during preeclampsia, such as endothelial cell dysfunction, hemodynamic abnormalities, and insulin resistance may contribute to VTE occurrence during and after delivery.21)22)
In diabetes, high blood glucose promotes vascular inflammation leading to increased risk of a CV event. Regarding study on the association between diabetes and VTE, a population-based case-control study, based on Rochester Epidemiology Project data from 1976 to 2000, showed that DM and diabetes complications are not independent risk factors for incident VTE.12) The theoretical mechanism that leads to the development of VTE, is that the atherosclerotic risk factors of diabetes, infection and hypercoagulability, affect VTE development. Many studies have revealed the association of diabetes with VTE and PE,23)24) and results of a recent meta-analysis that diabetes increased the risk for VTE 1.42-fold (95% CI, 1.12-1.77) support the association.25) A register-based case-control study in Norway also showed that GDM increased antenatal VTE by 4.0-fold (95% CI, 2.0-8.9).1) Interestingly, they did not find that GDM predicts postnatal VTE, suggesting that antepartum and postpartum VTE may involve different developmental mechanisms. Therefore, large differences exist between the unadjusted and adjusted odd ratios for all postnatal risk factors (preeclampsia, placenta previa, placenta abruptio, ART, and C-section). In this study, age and multiple pregnancies, which have been thought to be risk factors for VTE in pregnancy, demonstrated no association with postnatal VTE.
Our study reconfirmed the known risk factors of VTE in pregnancy, including the strong relationship between PIH and VTE in pregnancy, and found another new risk factor, placenta previa. Since in this study, age and GDM were not risk factors for VTE in pregnancy, if our results are confirmed, this finding could help reduce unnecessary diagnostic tests and the use of thromboprophylaxis. Even though thromboprophylaxis during pregnancy is relatively safe, bleeding, osteoporosis, thrombocytopenia, skin necrosis, and other adverse effects frequently continue to occur after anticoagulation therapy.26-28) More research is needed to produce better guidelines for thromboprophylaxis.
Our study has many limitations. Most importantly, this study was performed in one center and had a very small number of patients. Because the frequency of VTE is so low, it is difficult to conduct an association study in a single center; therefore, multicenter registry studies are needed. Another limitation is that our study did not consider the difference between antepartum and postpartum VTE. In our study, we had only five cases of antepartum VTE, and the remaining 22 cases developed in the postpartum period. Therefore, our results are more relevant to postpartum VTE, and more cases are needed to clarify VTE differences occurring before and after delivery, because ante- and postpartum risk patterns are different. Moreover, the diagnosis of DVT during pregnancy is generally hard to make. Since dyspnea, tachypnea, swelling, and discomfort in the legs are common during pregnancy and sometimes go without treatment, the incidence of actual DVT seems to be underestimated. This is why the incidence of DVT in our study was so low, and more aggressive effort should be devoted to the diagnosis of DVT in a larger study.
In conclusion, our study revealed that PIH, but not GDM, was associated with VTE in pregnancy. However, a large-scale, multicenter registry study is still required to determine the relationship between GDM and VTE.
Figures and Tables
 | Fig. 1Odds ratios of risk factors for venous thromboembolism in pregnancy and the puerperium. GDM: gestational diabetes mellitus, PIH: pregnancy-induced hypertension, ART: assisted reproduction technology. |
References
1. Jacobsen AF, Skjeldestad FE, Sandset PM. Incidence and risk patterns of venous thromboembolism in pregnancy and puerperium: a register-based case-control study. Am J Obstet Gynecol. 2008. 198:233.e1–233.e7.
2. Marik PE, Plante LA. Venous thromboembolic disease and pregnancy. N Engl J Med. 2008. 359:2025–2033.
3. Greer IA. Thrombosis in pregnancy: maternal and fetal issues. Lancet. 1999. 353:1258–1265.
4. Moon HJ, Rhim CY, Kim GW, et al. Risk factors of deep vein thrombosis and pulmonary embolism in Korean. Korean Circ J. 2005. 35:474–479.
5. Kim KY, Moon KW, Jeon DS, et al. A case of hereditary antithrombin III defiency manifestation of infarct and deep vein thrombosis. Korean Circ J. 2002. 32:521–525.
6. Lee SW, Seo HS, Ghil JH, Rim SJ, Kang SM, Chung NS. A case of internal Jugular vein thrombosis after in vitro fertilization. Korean Circ J. 2004. 34:214–219.
7. Lindqvist P, Dahlback B, Marsal K. Thrombotic risk during pregnancy: a population study. Obstet Gynecol. 1999. 94:595–599.
8. Ros HS, Lichtenstein P, Bellocco R, Petersson G, Cnattingius S. Pulmonary embolism and stroke in relation to pregnancy: how can high-risk women be identified? Am J Obstet Gynecol. 2002. 186:198–203.
9. Report of the National High Blood Pressure Education Program Working group report on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000. 183:S1–S22.
10. Cheung NW, Byth K. The population health significance of gestational diabetes. Diabetes Care. 2003. 26:2005–2009.
11. James AH, Jamison MG, Brancazio LR, Myers ER. Venous thromboembolism during pregnancy and the postpartum period: incidence, risk factors, and mortality. Am J Obstet Gynecol. 2006. 194:1311–1315.
12. Heit JA, Leibson CL, Ashrani AA, Petterson TM, Bailey KR, Melton LJ 3rd. Is diabetes mellitus an independent risk factor for venous thromboembolism? Arterioscler Thromb Vasc Biol. 2009. 29:1399–1405.
13. Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982. 144:768–773.
14. Koonin LM, MacKay AP, Berg CJ, Atrash HK, Smith JC. Pregnancy-induced mortality surveillance: United States, 1987-1990. MMWR CDC Surveill Summ. 1997. 46:17–36.
15. Brill-Edwards P, Ginsberg JS, Gent M, et al. Safety of withholding heparin in pregnant women with a history of venous thromboembolism. N Engl J Med. 2000. 343:1439–1444.
16. Pabinger I, Grafenhofer H, Kaider A, et al. Risk of pregnancy-associated recurrent venous thromboembolism in women with a history of venous thrombosis. J Thromb Haemost. 2005. 3:949–954.
17. Royal College of Obstetricians and Gynaecologists (RCOG). Thromboprophylaxis during pregnancy, labour and after vaginal delivery. Guideline No. 37. 2004. London: RCOG Press.
18. Royal College of Obstetricians and Gynaecologists (RCOG). Report of the Royal College of Obstetricians and Gynaecologists (RCOG) working party on prophylaxis against thromboembolism in gynaecology and obstetrics. 1995. London: RCOG Press.
19. Gherman RB, Goodwin TM, Leung B, Byrne JD, Hethumumi R, Montoro M. Incidence, clinical characteristics, and timing of objectively diagnosed venous thromboembolism during pregnancy. Obstet Gynecol. 1999. 94:730–734.
20. Kestenbaum B, Seliger SL, Easterling TR, et al. Cardiovascular and thromboembolic events following hypertensive pregnancy. Am J Kidney Dis. 2003. 42:982–989.
21. Roberts RN, Henriksen JE, Hadden DR. Insulin sensitivity in pre-eclampsia. Br J Obstet Gynaecol. 1998. 105:1095–1100.
22. Morris NH, Eaton BM, Dekker G. Nitric oxide, the endothelium, pregnancy and pre-eclampsia. Br J Obstet Gynaecol. 1996. 103:4–15.
23. Tsai AW, Cushman M, Rosamond WD, Heckbert SR, Polak JF, Folsom AR. Cardiovascular risk factors and venous thromboembolism incidence. Arch Intern Med. 2002. 162:1182–1189.
24. Petrauskiene V, Falk M, Waernbaum I, Norberg M, Eriksson JW. The risk of venous thromboembolism is markedly elevated in patients with diabetes. Diabetologia. 2005. 48:1017–1021.
25. Ageno W, Becattini C, Brighton T, Selby R, Kamphuisen PW. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation. 2008. 117:93–102.
26. James AH. Thromboembolism in pregnancy: recurrence risks, prevention and management. Curr Opin Obstet Gynecol. 2008. 20:550–556.
27. Santoro R, Iannaccaro P, Prejanò S, Muleo G. Efficacy and safety of the long-term administration of low-molecular-weight heparins in pregnancy. Blood Coagul Fibrinolysis. 2009. 20:240–243.
28. Spyropoulos AC, Turpie AG, Dunn AS, et al. Clinical outcomes with unfractionated heparin or low-molecular-weight heparin as bridging therapy in patients on long-term oral anticoagulants: the REGIMEN registry. J Thromb Haemost. 2006. 4:1246–1252.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download