Abstract
Paraplegia secondary to spinal cord infarction is a recognized complication of open thoracic and thoracoabdominal aortic aneurysm (TAA) repair. TAA is serious and unpredictable condition. Therefore, aortic repair requires thorough information on managing the potential complications will facilitate improve control the problem. We report the symptoms and management of paraplegia in a patient who underwent stent insertion as TAA.
Until the last 5 to 10 years, patients with aneurysm of the thoracoabdominal aorta had only one treatment option: open surgical repair. For patients who could not tolerate surgery due to co-morbidities, they faced the certainty of continued aneurysm enlargement and eventual rupture.1) Endovascular techniques are emerging as an alternative approach in the treatment of aortic conditions, and this approach offers less invasive therapy, particularly to patients classified as high-risk.2) Despite these advances, paraplegia secondary to spinal cord infarction is a recognized complication of thoracoabdominal aortic aneurysm (TAA) repair.3-5) We report our experience of stent graft treatment for TAA, with a focus on complication management.
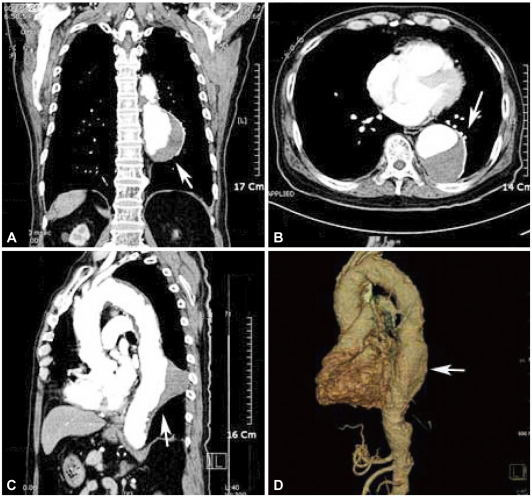
A 76-year-old male was diagnosed with lung cancer without histological confirmation. He was found to have developed a thrombosed aneurysm in the descending thoracic aorta. Thoracic CT scan revealed eccentric aneurysm of descending thoracic aorta with a length of 10 cm, diameter 6.5 cm, filled partially by thrombus (Fig. 1).
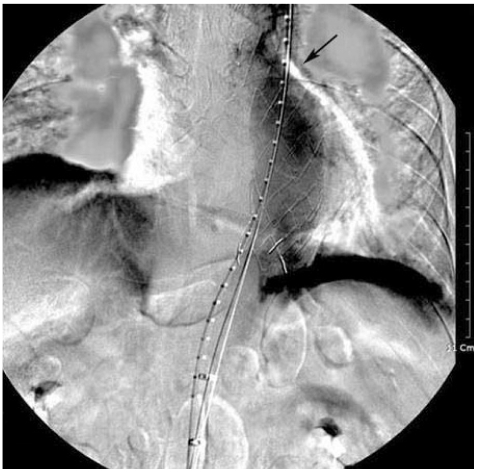
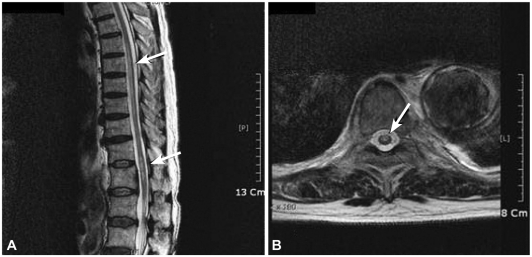
The decision was made, considering his general condition and underlying lung cancer, to repair this aneurysm using an endovascular graft. The procedure was performed in the angiography room using regional anesthesia. Femoral artery access was obtained bilaterally via cut downs. The right femoral artery underwent uneventful retrograde cannulation, measurements were performed, and a 40 mm diameter ×12 cm length stent was successfully inserted (SEAL S&G BIOTEC. Seongnam, Korea) distally.6-8) Completion arteriogram revealed a widely patent endovascular graft with patent descending thoracic aorta (Fig. 2). There was no evidence of proximal or distal endo-leakage. The procedure took approximately 3 hours in total, which remained well tolerated and the patient remained normotensive (systolic blood pressure 140-110 mmHg, diastolic blood pressure 110-70 mmHg), and the urine output was maintained constantly throughout. He complained of absent motor function in the extensor and flexor of the hip and both legs, especially on the left side 3 days after the procedure despite his blood pressures were well maintained during and after the procedure (systolic blood pressure 140-100 mmHg, diastolic blood pressure 110-65 mmHg). The motor grade of ankle dorsiflexors was estimated to be approximately 1/5, plantar flexors 1/5 and hallucis longi 2/5. Pinprick and temperature sensation were intact at the T9-T10 level with normal toe proprioception. An emergency MRI was obtained and the findings were consistent with spinal cord ischemia at T6-12, with elongated bright signal lesion on T2WI in mainly gray matter location (Fig. 3). These findings, along with abnormal neurologic examinations, were consistent with the anterior spinal artery syndrome.9)
The patient was managed with systemic steroid therapy, spinal drainage and pharmacologic support of blood pressure. The patient's mean arterial pressure was maintained using vasopressor at 75-85 mmHg and the lumbar cerebrospinal fluid (CSF) was drained in 10 mL aliquots to maintain CSF 12 mmH2O or less. Glucocorticoid therapy was administered in bolus at 30 mg/kg over 15 minutes, with maintenance infusion of 5.4 mg/kg for 48 hours.1)3)8)10-12) On post-procedure day 8, the CSF catheter was disconnected. His motor strength slowly showed improvement but his ankle flexion strength was 2/5 on the right, and 3/5 on the left. The patient was discharged on post-procedure day 40 with residual neurologic deficit. Two months later, he gained nearly full knee and ankle motor strength at 4/5 in both legs.
Spinal cord ischemia following elective repair of aortic aneurysm has been reported with an incidence of 0.25-0.3%.4) Spinal cord ischemia has been reported to occur following endovascular repair of thoracic aorta aneurysm, albeit with a lower incidence.13) Many factors have been implicated in the etiology of spinal cord ischemia. Prolonged suprarenal clamping, hypotension, thromboembolism, and interference of radicular artery of Adamkiewicz and the pelvic collateral circulation have all been considered.3)4)9) Whatever the mechanism, the final pathway of spinal cord ischemia is secondary to alteration of blood supply. The anterior spinal artery supplies approximately three-quarters of blood to the spinal cord, and two posterior arteries supply the remainder.4)9) The distal portion of the spinal cord also receives blood from the iliosacral and lumbosacral arteries, which contribute to the arterial network around the conus of the cord. In the thoracolumbar area, the anterior spinal artery receives blood from the artery of Adamkiewicz. In an anatomic study, this artery was found to originate from an intercostal artery from T5-T7 in 7%, T8-T12 in 82%, and from a lumbar artery of L1-L2 in 11% of the cadaver studied.4)
Occlusion of the artery of Adamkiewicz significantly disrupt the blood supply to the anterior spinal artery, and can potentially result in spinal cord infarction. Infarction in the distribution of the anterior spinal artery results in paresis, and loss of pain and temperature sensation with preservation of the posterior column function of vibration and proprioception.
In summary, paraparesis and paraplegia are rare and devastating complications known to occur in less than 0.5% of patients who undergo TAA repair. The advantage of endovascular repair holds promise for reducing perioperative morbidity, but as this case demonstrates, endovascular repair has not eliminated one of the most fearful complication.4)14)
References
1. Sullivan TM, Sundt TM 3rd. Complications of thoracic aortic endografts: spinal cord ischemia and stroke. J Vasc Surg. 2006; 43(Suppl A):85A–88A.
2. Fattori R, Napli G, Lovato L, et al. Descending thoracic aortic diseases: stent-graft repair. Radiology. 2003; 229:176–183. PMID: 12902611.
3. Cheung AT, Pochettino A, McGarvey LM, et al. Strategies to manage paraplegia risk after endovascular stent repair of descending thoracic aortic aneurysms. Ann Thorac Surg. 2005; 80:1280–1289. PMID: 16181855.
4. Garcia ND, Tehrani H, Morasch M, Pearce W, Matsumura J. Spinal cord ischemia following endovascular repair of an infrarenal aortic aneurysm. Ann Vasc Surg. 2002; 16:509–512. PMID: 12134217.
5. Berg P, Kaufmann D, Marrewijk CJ, Buth J. Spinal cord ischaemia after stent-graft treatment for infra-renal abdominal aortic aneurysms: analysis of the Eurostar database. Eur J Vasc Endovasc Surg. 2001; 22:342–347. PMID: 11563894.
6. Choi JH, Lim C, Park KH, Chung ES, Kang SG, Yoon CJ. Percutaneous endovascular stent-graft treatment for aortic disease in high risk patients: the early and mid-term results. Korean J Thorac Cardiovasc Surg. 2008; 41:239–246.
7. Ahn CM, Choi DH, Shim WH. Endovascular abdominal aortic aneurysmal repair: a Korean perspective. Korean Circ J. 2007; 37:459–463.
8. Gowda RM, Misra D, Tranbaugh RF, Ohki T, Khan IA. Endovascular stent grafting of descending thoracic aortic aneurysms. Chest. 2003; 124:714–719. PMID: 12907563.
9. Zuber WF, Gasper MR, Rothschild PD. The anterior spinal artery syndrome: a complication of abdominal aortic surgery: report of five cases and review of the literature. Ann Surg. 1970; 172:909–915. PMID: 5477667.
10. Bracken MB. Steroids for acute spinal cord injury. Cochrane Database Syst Rev. 2002; (3):CD001046. PMID: 12137616.
11. Weigang E, Hartert M, von Samson P, et al. Thoracoabdominal aortic aneurysm repair: interplay of spinal cord protecting modalities. Eur J Vasc Endovasc Surg. 2005; 30:624–631. PMID: 16023390.
12. Lim KJ, Kwan TD, Jeon YS, Kim JS. Anesthetic management of endovascular stent graft placement for thoracic aortic diseases: a case report. Korean J Anesthesiol. 2005; 49:106–110.
13. Grabenwöger M, Hutschala D, Ehrlich MP, et al. Thoracic aortic aneurysms: treatment with endovascular self-expandable stent grafts. Ann Thorac Surg. 2000; 69:441–445. PMID: 10735678.
14. Piffaretti G, Tozzi M, Lomazzi C, Rivolta N, Caronno R, Castelli P. Complications after endovascular stent-grafting of thoracic aortic diseases. J Cardiothorac Surg. 2006; 1:26. PMID: 16968547.
Fig. 1
A 76-year-old man presented with chronic aortic aneurysm. A and B: enhanced CT scan shows thrombosed saccular aneurysm of the descending thoracic aorta (white arrow). C and D: CT scan sagittal view shows atherosclerosis of the aorta and thrombosed dissection (white arrow).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download