This article has been retracted. See "Retraction: Aortic Dissection and Rupture in a Child" in Volume 42 on page 511.
Abstract
After developing sudden severe chest pain, an 11-year-old boy presented to the emergency room with chest pain and palpitations and was unable to stand up. The sudden onset of chest pain was first reported while swimming at school about 30 minutes prior to presentation. Arterial blood pressure (BP) was 150/90 mmHg, heart rate was 120/minute, and the chest pain was combined with shortness of breath and diaphoresis. During the evaluation in the emergency room, the chest pain worsened and abdominal pain developed. An aortic dissection was suspected and a chest and abdomen CT was obtained. The diagnosis of aortic dissection type B was established by CT imaging. The patient went to surgery immediately with BP control. He died prior to surgery due to aortic rupture. Here we present this rare case of aortic dissection type B with rupture, reported in an 11-year-old Korean child.
Though aortic dissection is very rare in young children,1) this abnormality is certainly recognized, particularly in those with congenital heart disease, connective tissue disorders, or severe trauma.2) Early diagnosis and treatment is crucial for this life-threatening abnormality.3) Aortic dissection type B is defined as the appearance of a false lumen at the segment distal to the left subclavian artery. This type of dissection occasionally extends toward the descending thoracic aorta and the abdominal aorta. Fatal complications of type B aortic dissection include rupture of the thoracic aorta, leg ischemia, visceral ischemia, and renal failure.1-4) Most frequently the presenting complaint is severe pain, and the report of the pain migrating is the most important clue for diagnosis.5)
An 11-year-old boy presented to the emergency department with severe chest pain that started while he was swimming at school, 30 minutes prior to presentation. This sudden onset of chest pain was the initial event, and was associated with shortness of breath and diaphoresis. The patient was obese, weighing 59 kg, over the 95th percentile for age. There was no history of trauma. The past medical history was significant for IgA nephropathy three years ago. The patient took medications, including an angiotensin converting enzyme-inhibitor and corticosteroids for the past three years, and antithrombotics for the past four months.
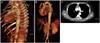
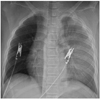
The physical examination revealed rapid and shallow heart sounds. The heart rate was 120/minute, the initial blood pressure (BP) was high, 150/90 mmHg in both arms, the respiration rate was 25/minute, and the body temperature was 36.8℃. Laboratory testing revealed a hemoglobin of 13.4 g/dL; a platelet count of 446,000/uL; Cardiac enzymes and serum electrolytes including Na+, K+, Ca++, phosphate were all normal. The electrocardiogram showed sinus tachycardia. The chest X-ray was normal (Fig. 1). The trans-thoracic echocardiography revealed a normal cardiac structure with normal systolic and diastolic function. To decrease the high BP and rapid heart rate, an IV beta-blocker was infused. The patient had no history of hypertension. Despite stabilizing the BP and heart rate, the sharp chest pain persisted at the mid-sternum and extended to the abdomen. Thus an aortic dissection was suspected and emergency computed tomography (CT) scanning of the chest and abdomen was performed. The diagnosis of aortic dissection was established by CT imaging from the visualization of a torn intimal flap noted from the left subclavian artery to the left common iliac artery (Fig. 2A). There was impending luminal obstruction of the left iliac artery due to the extension of the dissection (Fig. 2B and C). Treatment with intravenous esmolol and nitroprusside was started in the intensive care unit and his BP improved to 120/75 mmHg, the heart rate was normalized to 75/minute, and the chest pain was controlled with an intravenous morphine infusion. The patient was sent to surgery immediately. While waiting for the emergency operation, the eyes deviated and seizures developed with loss of consciousness. A cardiac arrest followed. A chest X-ray, taken immediately, demonstrated left lung haziness (Fig. 3) which was likely due to the sudden rupture of the aortic dissection.
An aortic dissection is a condition with many predisposing factors, and rarely occurs in children or adolescents. Fikar reported a retrospective analysis of aortic dissection and found that 22% of young children and adolescent patients had no apparent risk factors.1) This fatal abnormality certainly does occurs in young populations, particularly those with congenital heart disease, connective tissue disorders or severe trauma2); congenital cardiovascular disorders are the most common related conditions and trauma-associated aortic dissection is considered a relatively rare event.1)
Aortic dissection is a life-threatening condition that requires rapid diagnosis and treatment.3) Fikar reported that aortic dissections occur in less than 3.5% of the population, with a mortality of 0.035% in the young, under 19 years of age.4)5) The mortality rate of untreated aortic dissection rises by 1-2% hourly for the first 48 hours from symptom onset. Early surgery is associated with a better outcome.4) In addition, evaluation of predisposing conditions is important. Coarctation of the aorta and congenital aortic valve stenosis with bicuspid or quadricuspid aortic valves are the most common predisposing cardiovascular anomalies of aortic dissection. Marfan's syndrome, type IV Ehlers Danlos syndrome, and Turner's syndrome, are connective tissue disorders that are non-cardiac causes of aortic dissection. These disorders usually have clear physical stigmata associated and are inherited in an autosomal dominant fashion.4)
When it occurs in youth, aortic dissection usually arises during the adolescent period rather than childhood.6) In young children, rarer causes of aortic dissection have been reported, such as: trauma, cocaine abuse, and weight lifting.4) The most frequent presenting symptom of aortic dissection is severe pain (85-95%), the initial site of which varies, and frequently migrates in 60-80% to the exact site of extension of the dissection. Chest pain can involve the anterior and posterior chest wall, neck, jaw, shoulder, abdomen or extremities. The finding of migrating pain is the most valuable clue for diagnosis. Neurological symptoms affect 15-45% of patients; with confusion, weakness, and dysesthesias.5) High BP is an important distinctive finding when comparing children with adults, as hypertension is rare in children.2)4)
In this child's case, there are a few factors which may have contributed to the lack of prior symptoms. Steroid medication taken during the previous three years for treatment of IgA nephropathy might have masked the development of hypertension. Also, the steroid treatment probably had an influence on the disintegration of the connective tissue of the media; as for the cystic medial degeneration, it might have progressed and been associated with the development of hypertension.7) In addition, this child was obese, weighing 59 kg, over the 95th percentile for his age. In this particular case, the aortic dissection might have been related to the masked hypertension. In addition, the obesity (weight >95% for his age) might have been a causal factor of the hypertension.7) There were no findings to suggest a connective tissue disorder.
The aortic dissection was likely associated with obesity and hypertension, and aggravated by strenuous exercise (swimming), which led to the fatal event. Hatzaras et al.8) reported in 2007 that strenuous physical activity and severe emotional stress were clear precipitating factors of the acute onset of the thoracic pain of acute aortic dissection. Thus, severe physical and emotional stress may precipitate aortic dissection, presumably on the basis of a transient, severe hypertensive reaction. Strenuous physical activities include lifting weights, swimming, and shoveling snow.8) Edwin, in 2010, reported that swimming could precipitate acute aortic dissection in the absence of any predisposing factors; possibly this child's swimming triggered the arterial dissection, as reported previously.9)
The chest X-ray of an aortic dissection is not always specific for the aortic shadow. However, mediastinal widening, pleural effusion, abnormal aortic contour and cardiomegaly can be seen.4)5) Even though the patient had a normal chest X-ray, an aortic dissection was not excluded.11) Aortography, magnetic resonance imaging, or echocardiography are helpful, but the CT scan is the first-choice diagnostic modality. Transesophageal echocardiography is known to be highly sensitive and specific for detection of intimal flap aortic dissection as well and it is therefore regarded as the imaging modality of choice at some cardiovascular centers.4) It can also identify pericardial effusions, aortic insufficiency, and compromise of the coronary arteries; in addition it can be performed at the bedside in the emergency room.
Aortic dissection is categorized as two types: type A involves the ascending aorta, and type B has restricted involvement within the aorta distal to the left subclavian artery.11) Type B aortic dissection frequently precedes aneurysm formation and late rupture. Moreover, type B aortic dissection is usually associated with hypertension. In this case, though, there was no documented history of hypertension before the fatal event, nor were there symptoms or signs of hypertension. The aortic dissection might still have been associated with the hypertension, triggered by strenuous exercise (swimming).
In general, patients with aortic dissection may present with severe chest and back pain, leg ischemia, and lower-extremity neurological symptoms.12) In these cases, medical therapy could be recommended for uncomplicated type B aortic dissection. The first goal in medical treatment of an aortic dissection is to maintain the BP and heart rate.13) Surgery is necessary for type A dissection or type B dissection with impending rupture, rapid progression of symptoms, or risk of abnormal perfusion of vital organs.14-20)
The various aortic diseases which lead to dissection or rupture, cardiomyopathy, coronary anomalies, obstructive coronary artery disease, valvular disease, and myocarditis, are among the most common causes of cardiovascular deaths in the young.19) Many sudden deaths in the young occur during or shortly after exercise.19) Clinical suspicion of aortic dissection in children is based on the history of pain, which can be migratory. Hypertension may or may not be associated with aortic dissection in children. A normal chest X-ray cannot rule out the diagnosis of an aortic dissection; this grave condition can easily slip under the list of differentials when diagnosing children and adolescents. In conclusion, physicians working in an acute care setting, particularly in the emergency room, should be aware of the disorders and clinical characteristics predisposing patients to an acute aortic dissection, even in children and adolescents.4)
Figures and Tables
References
1. Fikar CR, Fikar R. Aortic dissection in childhood and adolescence: an analysis of occurrence over a 10-year interval in New York State. Clin Cardiol. 2009. 32:E23–E26.
2. Cooper DR, Lucke WC, Moseson DL. Aortic dissection in adolescence. Am Fam Physician. 1986. 34:137–142.
3. Horwitz AE, Benz-Bohm G, Heuser L, Crespo E, Dalichau H. Aortic dissection in childhood: occurrence and diagnostic procedure. Monatsschr Kinderheilkd. 1986. 134:28–31.
4. Fikar CR, Koch S. Etiologic factors of acute aortic dissection in children and young adults. Clin Pediatr (Phila). 2000. 39:71–80.
5. Fikar CR, Amrhein JA, Harris JP, Lewis ER. Dissecting aortic aneurysm in childhood and adolescence: case report and literature review. Clin Pediatr (Phila). 1981. 20:578–583.
6. Teien D, Finley JP, Murphy DA, Lacson A, Longhi J, Gillis DA. Idiopathic dilatation of the aorta with dissection in a family without Marfan syndrome. Acta Paediatr Scand. 1991. 80:1246–1249.
7. Mitsnefes MM. Hypertension in children and adolescents. Pediatr Clin North Am. 2006. 53:493–512.
8. Hatzaras IS, Bible JE, Koullias GJ, Tranquilli M, Singh M, Elefteriades JA. Role of exertion or emotion as inciting events for acute aortic dissection. Am J Cardiol. 2007. 100:1470–1472.
9. Edwin F, Aniteye EA, Sereboe L, Frimpong-Boateng K. Acute aortic dissection in the young: distinguishing precipitating from predisposing factors. Interact Cardiovasc Thorac Surg. 2009. 9:368.
10. Gray J, McCaw T, McGovern S. Spontaneous chest pain in a 14-year-old boy. Eur J Emerg Med. 2005. 12:253–254.
11. Rizzoli G, Scalia D, Casarotto D, Tiso E. Aortic dissection type A versus type B: a different post-surgical death hazard? Eur J Cardiothorac Surg. 1997. 12:202–208.
12. Schor JS, Yerlioglu ME, Galla JD, Lansman SL, Ergin MA, Griepp RB. Selective management of acute type B aortic dissection: long-term follow-up. Ann Thorac Surg. 1996. 61:1339–1341.
13. Hashimoto A, Kimata S, Hosoda S. Acute aortic dissection: a comparison between the results of medical and surgical treatments. Jpn Circ J. 1991. 55:821–823.
14. Cho SH, Sung K, Park KH, et al. Midterm results of aortic arch replacement in a Stanford type A aortic dissection with an intimal tear in the aortic arch. Korean Circ J. 2009. 39:270–274.
15. Qureshi SA. Use of covered stents to treat coarctation of the aorta. Korean Circ J. 2009. 39:261–263.
16. Park SH, Park HS, Lee JH, et al. A case of coronary artery dissection after aortic replacement in acute type A aortic dissection. Korean Circ J. 2009. 39:428–423.
17. Lee S, Kim W, Hwang SH, et al. The relationship of inflammatory reaction with the mortality of type B acute aortic syndrome. Korean Circ J. 2006. 36:387–392.
18. Kang WC, Joung BY, Ko YG, et al. Favorable outcome of endovascular stent-graft implantation for Stanford type B aortic dissection. Korean Circ J. 2003. 33:457–464.
19. Fishbein MC. Cardiac disease and risk of sudden death in the young the burden of the phenomenon. Cardiovasc Pathol. 2009. [Epub ahead of print].
20. DeSanctis RW, Doroghazi RM, Austen WG, Buckley MJ. Aortic dissection. N Engl J Med. 1987. 317:1060–1067.