Abstract
Background and Objectives
Although recent lipid-lowering therapies are effective in reducing low density lipoprotein-cholesterol (LDL-C) levels, many patients treated with lipid-lowering agents do not achieve target LDL-C levels, especially in very high risk patients. The aim of this study is to compare the effect of ezetimibe/simvastatin 10/20 mg and atorvastatin 20 mg on achieving a target LDL-C goal in very high risk patients.
Subjects and Methods
A total of 74 patients with very high risk were enrolled in the study. Very high risk patients were defined as patients that displayed established cardiovascular disease with multiple major risk factors, poorly controlled risk factors, multiple risk factors of the metabolic syndrome and acute coronary syndromes. Patients were randomized into two groups: ezetimibe/simvastatin 10/20 mg (n=36) and atorvastatin 20 mg (n=38). Follow-up lipid profile was obtained 6 weeks later. A target goal of LDL-C was defined as less than 70 mg/dL at follow-up.
Results
Baseline clinical and laboratory data were similar between the two groups. Achieving a target LDL-C goal was observed in 41.7% of Group 1 and 44.7% of Group 2 at 6 weeks (p=0.82). Changes in other lipid profiles were not significantly different but the tolerability of the two groups was similar.
Go to : 
The importance of lowering low density lipoprotein-cholesterol (LDL-C) levels to prevent major cardiovascular problems has been well known and current guidelines emphasize the need to reduce LDL-C to target levels.1)2) Moreover, in high-risk or moderately high-risk persons, it is advised that the intensity of therapy be sufficient to achieve at least 30 to 40% reduction in LDL-C levels.3) Thus, more effective lipid-lowering therapies are needed to reach the established LDL-C goal. To attain optimal LDL-C levels, higher doses of more potent statins (3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors) provide greater reduction in LDL-C. Recently, the challenge of attaining more aggressive LDL-C goals has stimulated research into possible new combinations of lipid-lowering agents with complementary mechanisms of actions. The major effect of statin is to reduce LDL-C by inhibiting cholesterol synthesis. Ezetimibe is a novel cholesterol absorption inhibitor that prevents the absorption of cholesterol by inhibiting the passage of cholesterol of dietary and biliary origin across the intestinal wall.4)5) Clinical trials have shown that the co-administration of ezetimibe with simvastatin, a combination therapy that inhibits cholesterol biosynthesis and blocks its intestinal absorption, is more effective at lowering LDL-C than any one of the agents alone.6)7) Heart Protection Study (HPS)8) and the Pravastatin or Atorvastatin Evaluation and Infection Therapy trial9) suggested that cardiovascular event reduction may be obtained by reducing LDL-C levels to substantially below 100 mg/day. On the basis of data from 2 studies, National Cholesterol Education Program Adult Treatment Panel (NCEP ATP) III recommended that an LDL-C goal of <70 mg/dL is a reasonable clinical strategy when risk is very high.3)
The primary objective of the current trial was to compare the effect of ezetimibe/simvastatin 10/20 mg and atorvastatin 20 mg in achieving the target LDL-C goal of <70 mg/dL in subjects with very high risk. The secondary objective was to compare the effect of ezetimibe/simvastatin 10/20 mg with that of atorvastatin 20 mg on the lipid profile except LDL-C.
Go to : 
This single center, randomized, open-label study was designed to evaluate the efficacy and tolerability of ezetimibe/simvastatin 10/20 mg and atorvastatin 20 mg in very high risk patients. The protocol was approved by appropriate institutional review boards, and all patients provided written informed consent before initiation of any study procedure. Patients discontinued fibrate therapy for a 12 weeks wash-out period and all other lipid-lowering therapy 4 weeks before the start of the study. Patients were randomized after coronary angiography to ezetimibe/simvastatin 10/20 mg or atorvastatin 20 mg once daily before bedtime in a 1:1 ratio using a computer-generated random table. Patients were asked to count their unused medication at the last day of treatment and compliance was assessed by counting the remaining tablets.
Based on the previous study,10) with a sample size of approximately 36 patients per treatment arm, this study had 80% effectiveness to detect a 6.9% difference, assuming a standard deviation (SD) of 20.1% and a significance level of 0.05 (1-sided).
From February 2008 to October 2008, patients with coronary artery disease and documented hypercholesterolemia (LDL-C >100 mg/dL and ≤250 mg/dL) at screening were enrolled. Patients were 20 to 79 years of age. Very high risk patients were defined as those with the presence of established cardiovascular disease plus 1) multiple major risk factors (especially diabetes), 2) poorly controlled risk factors {especially continued cigarette smoking, uncontrolled blood pressure and low high density lipoprotein-cholesterol (HDL-C)}, 3) multiple risk factors of the metabolic syndrome {especially high triglycerides (TG) ≥200 mg/dL plus non HDL-C ≥130 mg/dL with low HDL-C (<40 mg/dL), impaired fasting glucose and central obesity} and 4) patients with acute coronary syndromes (ACS).3) Other criteria included fasting serum TG <400 mg/dL and aspartate aminotransferase (AST), alanine aminotransferase (ALT), or creatine kinase (CK) level ≤1.5 times the upper limit of normal levels.
Exclusion criteria included conditions or medications that could have affected lipid levels, such as patients with congestive heart failure defined by the New York Heart Association class III or IV, as well as patients with poorly controlled hypertension (systolic blood pressure >180 mmHg or diastolic blood pressure >100 mmHg), evidence of uncontrolled endocrine or metabolic disease known to influence serum lipid profile, and concomitant excluded drug use (i.e. immunosup-pressants, corticosteroids, or potent inhibitors of cytochrome P450 3A4).
Measurements of lipid parameters were performed randomly 6 weeks after treatment. Blood samples were taken after fasting for 12 hours. In patients with ACS, baseline blood samples were collected between 24 and 48 hours of diagnosis. The primary efficacy end point was the percentage of patients achieving the LDL-C goal (<70 mg/dL) of NCEP ATP III guideline after 6 weeks of treatment. Secondary efficacy end points included the percentage changes of the lipid profile such as total cholesterol (TC), TG, HDL-C, non HDL-C, apolipoprotein (Apo) B and high-sensitivity C-reactive protein (hs-CRP) as well as the ratio of LDL-C and HDL-C (LDL-C/HDL-C) at follow-up.
Tolerability assessment included the collection of adverse events, physical examination and vital signs. Laboratory measurements included AST, ALT, CK, blood urea nitrogen and creatinine. Pre-specified adverse events included those that were gastrointestinal-related and hepatitis-related adverse events as well as incidences of clinically significant elevations in AST and ALT, which were ≥3 times the upper limit of normal levels, and CK elevations, which were ≥5 times the upper limit of normal levels, with or without muscle symptoms.
Efficacy analyses of biological parameters were performed in patients who had taken at least one dose of randomized medications, had a baseline efficacy measurement and at least one efficacy measurement during the treatment period. The changes of each parameter were calculated from the mean±SD and differences were statistically assessed by an unpaired t-test. Proportions were compared by a chi-square test with 95% confidence intervals. All statistical tests were two-sided, with the level of significance being set at p<0.05.
Go to : 
A total of 85 patients were screened for inclusion in the study and randomized in similar numbers with ezetimibe/simvastatin 10/20 mg (n=42) or atorvastatin 20 mg (n=43). Of these, 36 patients (85.7%) in the ezetimibe/simvastatin group and 38 patients (88.4%) in the atorvastatin group successfully completed the 6 week treatment period. Eleven (12.9%) patients were discontinued from the study. Seven patients were lost at follow-up and four patients showed protocol violation.
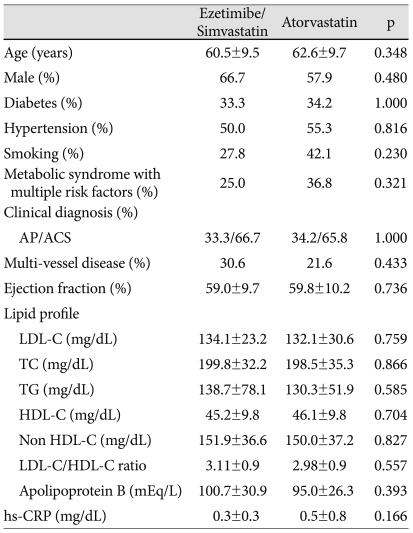
There were no clinically significant differences in baseline demographic or disease characteristics across the two treatment groups (Table 1). All patients received a loading dose of aspirin (300 mg) and clopidogrel (300 mg) before coronary angiography. During follow-up, the anti-platelet regimen was not different (83.3% in the ezetimibe/simvastatin group vs. 86.8% in the atorvastatin group in case of dual anti-platelet therapy, 11.1% in the ezetimibe/simvastatin group vs. 2.6% in the atorvastatin group, in case of triple anti-platelet therapy, p=0.194. Furthermore, beta blocker (83.3% in the ezetimibe/simvastatin group vs. 78.9% in the atorvastatin group, p=0.769) and angiotensin-converting enzyme inhibitor (77.8% in the ezetimibe/simvastatin group vs. 73.7% in the atorvastatin group, p=0.789) were similar between two groups. The majority of study participants presented ACS (66.7% in the ezetimibe/simvastatin group vs. 65.8% in the atorvastatin group, p=1.000). Among ACS patients, 1 patient in the ezetimibe/simvastatin group and 2 patients in the atorvastatin group took statin before enrollment. However, the washout period was at least a year. Baseline lipid variables were similar between the two groups.
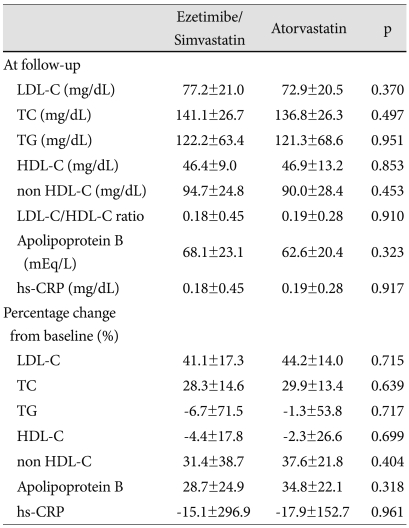
The incidence of achieving target LDL-C levels (<70 mg/dL) was not different between the two groups (41.7% in the ezetimibe/simvastatin group vs. 44.7% in the atorvastatin group, p=0.818). At follow-up, percent reduction of LDL-C level was 41.1% in the ezetimibe/simvastatin group and 44.2% in the atorvastatin group (p=0.715) (Table 2). For the secondary efficacy end points, both the ezetimibe/simvastatin and atorvastatin groups produced significant decrease from the baseline in TC (28.3% vs. 29.9%, p=0.639), TG (-6.7% vs. -1.3, p=0.717), HDL-C (-4.4% vs. -2.3%, p=0.699, non HDL-C (31.4% vs. 37.6%, p=0.404), Apo B (28.7% vs. 34.8%, p=0.318) and hs-CRP (-15.1% vs. -17.9%, p=0.961). Our results showed that the LDL-C/HDL-C ratio at follow-up was not different between the two groups (0.18 vs. 0.19, p=0.910).
Treatment with ezetimibe/simvastatin 10/20 mg and atorvastatin 20 mg was generally well tolerated and the mean compliance was over 99% in both groups. Overall, the prevalence of adverse events were 2.8% in the ezetimibe/simvastatin group and 5.3% in the atorvastatin group (p=1.000). One patient in the ezetimibe/simvastatin group (2.8%) had gastrointestinal trouble such as dyspepsia and abdominal pain. In the atorvastatin group, one patient (2.6%) had diarrhea and one (2.6%) showed elevation of AST and ALT, which was ≥3 times the upper limit of normal levels. No patient in either group had CK elevations of ≥5 times the upper limit of normal levels, with or without muscle symptoms.
Go to : 
On the basis of HPS and PROVE-IT-Thrombolysis In Myocardial Infarction (TIMI) 22, an LDL-C level of 100 mg/dL does not appear to be a threshold in high-risk patients and additional benefit may be obtained by reducing LDL-C levels to substantially below 100 mg/dL.11)12) NCEP ATP III reinforced an LDL-C level of 100 mg/dL to be a minimal goal of treatment of high-risk patients and an LDL-C goal of <70 mg/dL to be an optional goal for very high-risk patients.3)
The present study examined the lipid-lowering efficacy and tolerability of ezetimibe/simvastatin 10/20 mg and atorvastatin 20 mg in patients with very high-risk. The proportion of patients reaching an LDL-C level of less than 100 mg/dL was similar (86.1% in the ezetimibe/simvastatin group vs. 86.8% in the atorvastatin group). Achieving the target goal of <70 mg/dL was not statistically different between the two groups (41.7% in the ezetimibe/simvastatin group vs. 44.7% in the atorvastatin group). Also, the percentage reduction of LDL-C from baseline was similar (ezetimibe/simvastatin group 41% vs. atorvastatin group 44%). Both fulfilled the recommendation that intensity of therapy be sufficient to achieve at least a 30% to 40% reduction in LDL-C levels.13)
However, this study showed discrepancy in percentage reduction of LDL-C as compared with other studies although there was no similar study which compared the efficacy of lipid-lowering agents in very high-risk patients. The Vytorin Versus Atorvastatin (VYVA) study showed 50.6% reduction of LDL-C in ezetimibe/simvastatin 10/20 mg treatment and 43.7% reduction in atorvastatin 20 mg treatment.10) In the previous study which evaluated the efficacy of ezetimibe/simvastatin and atorvastatin in patients with type 2 diabetes mellitus and hypercholesterolemia, the percent change of LDL-C from baseline in ezetimibe/simvastatin 10/20 mg was 53.6% and atorvastatin 20 mg was 44.6%.14) Although the inclusion criteria in each study were different, the reduction of LDL-C in atorvastatin 20 mg was similar. However, the percentage reduction of LDL-C in ezetimibe/simvastatin 10/20 mg was greater than that of the present study. The most important and likely explanation may be the small number of patients. A different baseline of the characteristics of enrolled patients may also be related. Majority of patients in the present study were ACS (66.7% in ezetimibe/simvastatin group vs. 65.8% in atorvastatin group). The efficacy of atorvastatin in ACS was already proved by PROVE-IT-TIMI 22 study.12) However, the study regarding the efficacy of ezetimibe/simvastatin in ACS has still been investigated.15) The other explanation may be drug compliance, which was evaluated only by interviews with the patients.
The tolerability elicited by both drugs in the present study was consistent with previous reports.11)16-19) Overall incidence of adverse events was low in both groups.
This study evaluated the efficacy of ezetimibe/simvastatin 10/20 mg and atorvastatin 20 mg in a small number of very high-risk patients. Although this study did not show any difference in reaching target LDL-C goal as well as in reducing LDL-C from baseline after 6-week follow-up, it was useful because it was performed on Korean patients with very-high risk. This data revealed that ezetimibe/simvastatin 10/20 mg or atorvastatin 20 mg was still under the correct dose to patients with very-high risk including ACS. Therefore, more potent lipid-lowering therapy will be considered to achieve their LDL-C level goal.
This study has several limitations. Although the number of patients was calculated by LDL-C lowering effect of two drugs, the major limitation was the small number of patients. In addition, according to the VYVA study,10) the potency between ezetimibe/simvastatin 10/20 mg and atorvastatin 20 mg was not comparable in overall hypercholesterolemia although this result could not completely apply to Korean patients because of different disease characteristics and racial difference. Furthermore, almost all the enrolled patients were ACS and this could affect the change of lipid parameters. One important aspect of this study was that ezetimibe/simvastatin 10/20 mg and atorvastatin 20 mg did not attain the target LDL-C goal in more than half of the patients. A comparison of a higher dosage of ezetimibe/simvastatin and atorvastatin with a large population compared to the current study will be helpful to clarify the efficacy and tolerability in Korean patients with very high-risk.
In summary, ezetimibe/simvastatin 10/20 mg and atorvastatin 20 mg showed similar effectiveness in achieving a target LDL-C goal and lipid-lowering effect in Korean patients with very high risk, especially in ACS. Furthermore, a higher dose compared to ezetimibe/simvastatin 10/20 mg and atorvastatin 20 mg will be needed to attain a better rate of target LDL-C goal in this patient subset.
Go to : 
References
1. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001; 285:2486–2497. PMID: 11368702.
2. Kannel WB, Castelli WP, Gordon T. Cholesterol in the prediction of atherosclerotic disease. New perspectives based on the Framingham study. Ann Intern Med. 1979; 90:85–91. PMID: 217290.
3. Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004; 110:227–239. PMID: 15249516.
4. Van Heek M, France CF, Compton DS, et al. In vivo metabolism-based discovery of a potent cholesterol absorption inhibitor, SCH 58235, in the rat and rhesus monkey through the identification of the active metabolites of SCH48461. J Pharmacol Exp Ther. 1997; 283:157–163. PMID: 9336320.
5. Van Heek M, Farley C, Compton DS, et al. Comparison of the activity and disposition of the novel cholesterol absorption inhibitor, SCH 58235, and its glucuronide, SCH60663. Br J Pharmacol. 2000; 129:1748–1754. PMID: 10780982.
6. Davidson MH, McGarry T, Bettis R, et al. Ezetimibe coadministered with simvastatin in patients with primary hypercholesterolemia. J Am Coll Cardiol. 2002; 40:2125–2134. PMID: 12505224.
7. Goldberg AC, Sapre A, Liu J, Capece R, Mitchel YB. Ezetimibe Study Group. Efficacy and safety of ezetimibe coadministered with simvastatin in patients with primary hypercholesterolemia: a randomized, double-blind, placebo-controlled trial. Mayo Clin Proc. 2004; 79:620–629. PMID: 15132403.
8. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002; 360:23–33. PMID: 12114037.
9. Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus mode-rate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004; 350:1495–1504. PMID: 15007110.
10. Ballantyne CM, Abate N, Yuan Z, King TR, Palmisano J. Dose-comparison study of the combination of ezetimibe and simvastatin versus atorvastatin in patients with hypercholesterolemia: the Vytorin Versus Atorvastatin (VYVA) study. Am Heart J. 2005; 149:464–473. PMID: 15864235.
11. Collins R, Armitage J, Parish S, et al. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003; 361:2005–2016. PMID: 12814710.
12. Murphy SA, Cannon CP, Wiviott SD, et al. Effect of intensive lipid-lowering therapy on mortality after acute coronary syndrome (a patient-level analysis of the Aggrastat to Zocor and Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 trials). Am J Cardiol. 2007; 100:1047–1051. PMID: 17884359.
13. American Diabetes Association. Standards of medical care in diabetes: 2007. Diabetes Care. 2007; 30(Suppl 1):S4–S41. PMID: 17192377.
14. Goldberg RB, Guyton JR, Mazzone T, et al. Ezetimibe/simvastatin vs atorvastatin in patients with type 2 diabetes mellitus and hypercholesterolemia: the VYTAL study. Mayo Clin Proc. 2006; 81:1579–1588. PMID: 17165637.
15. Cannon CP, Giugliano RP, Blazing MA, et al. Rationale and design of IMPROVE-IT (IMProved Reduction of Outcomes: Vytorin Efficacy International Trial): comparison of ezetimbe/simvastatin versus simvastatin monotherapy on cardiovascular outcomes in patients with acute coronary syndromes. Am Heart J. 2008; 156:826–832. PMID: 19061694.
16. Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005; 366:1267–1278. PMID: 16214597.
17. Ballantyne CM, Blazing MA, King TR, Brady WE, Palmisano J. Efficacy and safety of ezetimibe co-administered with simvastatin compared with atorvastatin in adults with hypercholesterolemia. Am J Cardiol. 2004; 93:1487–1494. PMID: 15194018.
18. Davidson MH, Ballantyne CM, Kerzner B, et al. Efficacy and safety of ezetimibe coadministered with statins: randomised, placebo-controlled, blinded experience in 2382 patients with primary hypercholesterolemia. Int J Clin Pract. 2004; 58:746–755. PMID: 15372846.
19. Yun KH, Park HY, Choi JH, et al. Comparison of efficacy and safety after administering high potency statin to high risk patients: rosuvastatin 10 mg versus atorvastatin 20 mg. Korean Circ J. 2007; 37:154–160.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download