Abstract
Inadvertent left internal mammary artery (LIMA)-great cardiac vein (GCV) anastomosis is a rare complication of coronary artery bypass graft surgery. Patients with iatrogenic aortocoronary fistula (ACF) were usually treated surgical repair, percutaneous embolic occlusion with coil or balloon. We report a case of iatrogenic LIMA to GCV anastomosis successfully treated with coil embolization and protected left main coronary intervention through the percutaneous transfemoral approach.
Iatrogenic left internal mammary artery (LIMA)-great cardiac vein (GCV) fistula is an unusual complication of coronary artery bypass graft (CABG) surgery. About acquired aortocoronary fistula (ACF) of CABG , surgical repair has been generally recommended for ischemic symptoms regardless of shunt ratio.1)2) However, these days, depending on clinical symptoms and hemodynamic measurements, patients are treated conservatively,3) interventionally by embolization techniques with coil,4)5) by detachable balloon,6)7) or other surgical means.8) We report a patient with inadvertent LIMA-GCV fistula successfully treated with coil embolization using microcoils.
A 50-year-old man with a history of type 2 diabetes mellitus and old cerebral infarction was admitted to our cardiovascular center with a three-month history of chest pain associated with progressive effort and dyspnea on exertion. Coronary angiography revealed severe multiple vessel disease. He preferred CABG to multiple stents and underwent on-pump CABG. According to the operation record, the LIMA was anastomosed end to side with the mid left anterior descending coronary artery (LAD), and saphenous vein grafts were anastomosed to the left circumflex artery and right coronary artery. Although the patient was stable during the post-operative period, routine follow up coronary CT angiography demonstrated inadvertent LIMA-GCV anastomosis that filled the coronary sinus one week following the operation (Fig. 1). Follow-up echocardiography showed global hypokinesia of the left ventricle (LV) with mild LV systolic dysfunction that aggravated LV contractility compared to the last echocardiography. Coronary angiography demonstrated inadvertent LIMA-GCV anastomosis that filled the coronary sinus (Fig. 2).
Although the patient was stable, we decided to treat LIMA to GCV anastomosis because most ACF patients experience symptoms between 6 weeks and 4 years after CABG surgery.10) We decided to proceed protected left main coronary artery (LM) to proximal LAD by implanting a drug eluting stent (DES), and subsequently attempt to close the LIMA-GCV fistula by coil embolization via right transfemoral approach. We applied a 5 Fr 3.5 Judkins left coronary guiding catheter (COOK®, USA), and LM stenting was completed using a Taxus stent-3.5×38 mm-implantation (Boston scientific, USA) deployed at up to 20 atm with optimal angiographic results.
Iatrogenic LIMA-GCV fistula was occluded using three coils. Embolization materials were chosen to be 2 or 3 mm larger than the target vessel to prevent migration of these coils to the coronary sinus. Three coils (one Tornado Embolization Microcoil, MWCE-18S-4/2, two Micro Nester Embolization coil, MWCE-18-14-3; William Cook Europe, Denmark) were placed into the distal part of the LIMA graft via the percutaneous transfemoral approach. There was no residual shunt flow in the coronary sinus after coil embolization (Fig. 3). The patient was discharged 5 days later and remained medically stable one year later.
The internal mammary artery was used for the first time in 1951 when Vineberg reported an experimental technique to revascularize the cardiac muscle using the internal mammary artery (IMA) directly implanted to the myocardium.11) The first CABG in humans using IMA was performed by Longmire in 1958.12) Since the first CABG, at least 20 cases of acquired ACF have been reported in the medical literature. However, most of these cases involved the use of saphenous vein grafts13) and a few reported iatrogenic LIMA to coronary vein fistula. In 1996, Calkins et al.13) reported and reviewed the clinical manifestations, the physical findings and management of 18 patients with iatrogenic ACF who underwent CABG. According to this report, the presenting symptoms and signs in the postoperative period included angina, dyspnea, congestive heart failure, ventricular tachycardia and fatigue. The auscultatory findings (new onset continuous murmurs) and symptoms help to diagnose acquired ACFs. In all patients, the presence of an ACF was confirmed by coronary angiography which shows direct visualization of the involved graft and conduit.14) Most patients experience symptoms between 6 weeks and 4 years after CABG surgery. These data support therapeutic closures in all symptomatic patients and in asymptomatic patients with clinical findings of left to right shunt, because delayed symptoms appear in patients who have small, acquired, ACF and eventually admitted to hospital due to uncomfortable symptoms.
Treatment methods should be selected taking into account fistula size, vessel characteristics, symptoms intensity and safety. Several reports have demonstrated that surgical repair was safe and effective, had high survival and closure rate. However, after surgery, myocardial infarction and recurrence have been reported and repeat surgery involves repeated median sternotomy and sometimes cardiopulmonary bypass. It is therefore recommended that the majority of acquired ACF can and should be performed by percutaneous techniques initially, even if staged procedure is ultimately required and surgery should be limited to fistulae with large branch vessels that could be compromised within the embolization target area, or coronary lesions with multiple fistulous communication without a single, narrow restrictive drainage site into a cardiac chamber or vessels.10)
The use of percutaneous transcatheter techniques can reduce hospital stay, improve recovery time, eliminate the need for thoracotomy, and reduce cost, and result in safer intervention than surgery.10) However, balloon embolization require large sheaths and embolization can occur if there is premature balloon deflation. Coil embolization techniques are now the most widely used approach to occlude AVF, because it is more convenient and is associated with less complications than balloon embolizaion.16) However, it has been reported that only five cases of ACF have been performed successfully using coil embolization.13)15)17)18) Why is less successful coil embolizaiton of iatrogenic ACF cases seem to be first, migrated coil into coronary venous system or entering epicardial coronary arteries due to inappropriate coil size, location site and lack of ability to cannulating the distal fistula and second, maybe iatrogenic ACF underestimated.
We have demonstrated successful occlusion of iatrogenic ACF by creating an end to side anastomosis of LIMA to GCV using coil emboilization and revascularization LM to LAD stenosis with a DES through the percutaneous transfemoral approach. The recommendations for the treatment of acquired ACF have been not established until now. In the hands of experienced and skillful cardiologists, pecutaneous coil embolization is the most appropriate treatment for acquired ACF, which appears to be effective, safe and time sparing.
Figures and Tables
Fig. 1
Volume rendering images (A and B) and axial view (C and D) of coronary CT angiography showed inadvertent left internal mammary artery to great cardiac vein anastmosis (white arrow). LIMA: left internal mammary artery graft, GCV: great cardiac vein.
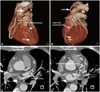
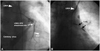
Fig. 2
Left internal mammary artery angiography showing the left internal mammary artery anastomsed to the great cardiac vein, with contrast filling and dilated coronary sinus emptying into right atrium. LIMA: Left internal mammary artery graft, GCV: great cardiac vein.

Fig. 3
A: left internal mammary artery angiography showed guide wire in LIMA and GCV. B: iatrogenic LIMA-GCV fistula was occluded using 3 coils (black arrows). There was no residual shunt flow in the iatrogenic LIMA-GCV fistula after coil embolization B. LIMA: left internal mammary
artery graft, GCV: great cardiac vein.
References
1. Balanescu S, Sangiorgi G, Castelvecchio S, Medda M, Inglese L. Coronary artery fistulas: clinical consequences and methods of closure. A literature review. Ital Heart J. 2001. 2:669–676.
2. Lawrie GM, Morris GC Jr, Winters WL. Aortocoronary saphenous vein autograft accidentally attached to a coronary vein: follow up angiography and surgical correction of the resultant arteriovenous fistula. Ann Thorac Surg. 1976. 22:87–90.
3. Scholz KH, Wiegand V, Rosemeyer P, Chemnitius JM, Kreuzer H. Aorto-coronary artery to coronary vein fistula with the potential of co-ronary steal as a complication of saphenous vein jump bypass graft. Eur J Cardiothorac Surg. 1993. 7:441–442.
4. Braun P, Holtgen R, Stroh E, et al. Coil embolization of an AV-fistula between the left thoracic artery and vein after coronary artery bypass surgery. Z Kardiol. 1999. 88:812–814.
5. Lopez JJ, Kuntz RE, Baim DS, Johnson RG, Kim D. Percutaneous occlusion of an iatrogenic aortosaphenous vein: coronary vein fistula via retrograde coronary sinus approach. Cathet Cardiovasc Diagn. 1996. 37:339–341.
6. Graeb DA, Morris DC, Ricci DR, Tyers GF. Balloon embolization of iatrogenic aortocoronary arteriovenous fistula. Cathet Cardiovasc Diagn. 1990. 20:58–62.
7. Peregrin JH, Zelizko M, Kovac J. Detachable balloon embolization of an iatrogenic aortocoronary arteriovenous fistula combined with aortocoronary bypass PTCA: a case report. Cathet Cardiovasc Diagn. 1992. 27:137–140.
8. Hubert JW, Thanavaro S, Ruffy R, Connors J, Oliver GC. Saphenous vein bypass to the posterior interventricular vein: an unusual complication of coronary artery surgery. South Med J. 1982. 75:1144–1146.
9. Maier LS, Buchwald AB, Ehlers B, Rühmkorf K, Scholz KH. Closure of an iatrogenic aortocoronary arteriovenous fisula: transcatheter balloon failed coil embolization and salvage of coils that migrated into coronary venous system. Catheter Cardiovasc Interv. 2002. 55:109–112.
10. Okubo M, Nykanen D, Benson LN. Outcomes of transcatheter embolization in the treatment of coronary artery fistulas. Catheter Cardiovasc Interv. 2001. 52:510–517.
11. Vineberg A, Miller G. Internal mammary coronary anastomosis in the surgical treatment of coronary artery insufficiency. Can Med Assoc J. 1951. 64:204–210.
12. Longmire WP Jr, Cannon JA, Kattus AA. Direct vision coronary endarterectomy for angina pectoris. N Engl J Med. 1958. 259:993–999.
13. Calkins JB Jr, Talley JD, Kim NH. Iatrogenic aortocoronary venous fistula as a compleication of coronary artery bypass surgery: patient report and review of literature. Cathet Cardiovasc Diagn. 1996. 37:55–59.
14. García-Rinaldi R, Marcano H. Inadvertent anasomosis of internal mammary artery to great cardiac vein. Tex Heart Inst J. 2010. 37:254–255.
15. White RW, Sivananthan MU, Kay PH. Aortocoronary bypass graft fistula after surgical treatment of circumflex coronary artery fistula: a unique variation of a rare condition successfully treated with percutaneous embolization. Interact Cardiovasc Thorac Surg. 2010. 10:256–257.
16. An HS, Kang TG, Yun HJ, et al. Hypertension caused by renal arteriovenous fistula. Korean Circ J. 2009. 39:548–550.
17. Martinez UR, Rivero RZ, Salgado CM, Buenrostro JM, Barcena JA. Images in cardiovascular medicine: iatrogenic internal mammary artery-to-great cardiac vein anastomosis. Circulation. 2006. 114:e359.
18. Pemberton J, Muir DF. Anastomosis of an internal mammary artery to anterior cardiac vein. Am J Cardiol. 2007. 100:337.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download