Abstract
Advances in percutaneous coronary intervention (PCI) and peri-procedural potent antithrombotic treatments during the past decade have dramatically improved the outcomes of ischemic heart disease. The femoral artery is the vascular route used in PCI in most catheterization labs. However, when the femoral artery is used as the approaching vessel, local hemorrhagic complication is not rare in the era of potent antithrombotics. Recent studies have suggested that peri-procedural bleeding complications after PCI are associated with increased short- and long-term morbidity and mortality. On the other hand, there has been growing interest in transradial PCI due to rare complications at the puncture site, patient conveniences, early discharge and shortened hospitalization periods. Furthermore, the indications of transradial PCI are expanding to the complex lesion subsets due to the miniaturization of devices used, improvement of devices and techniques, and accumulated experience with the use of transradial PCI. In this review, we discuss the data of transradial PCI as a potential default route in coronary artery interventions, as well as other issues that may raise concerns with transradial PCI.
Femoral approach has been the preferred vascular access for coronary angiography and percutaneous coronary intervention (PCI). However, recent reports have suggested that the radial artery approach for coronary angiography and interventions might be effective in terms of procedural outcomes compared to transfemoral approach and permits a wide range of diagnostic and therapeutic interventions. Furthermore, the transradial approach is a very safe alternative to femoral access due to a low rate of vascular entry site complications.1-3)
In the recent era of potent antiplatelet and anticoagulant therapy, post-PCI bleeding complications are closely related with an increase of in-hospital outcomes, as well as mid- and long-term mortality.4)5) Transradial coronary intervention (TRI) is an attractive access site for interventionists, compared to transfemoral coronary intervention (TFI), when considering the reduction of bleeding complications.
Although the amount of existing data comparing outcome after femoral and radial PCI is modest, a recent meta-analysis of 23 randomized trials showed that there were no significant differences in death, myocardial infarction (MI), or stroke between the transradial and transfemoral approach (TFI).6) However, the mortality rate tends to be lower in the transradial group [mortality rate: 1.2% vs. 1.8%, for TRI vs. TFI, odds ratio (OR): 0.74 {95% confidence interval (CI) 0.42-1.30}, p=0.29]. The rates of MI and stroke individually were both similar to the trend seen in mortality {MI: 2.0% vs. 2.9% for TRI vs. TFI, OR 0.76 (95% CI 0.49-1.17), p=0.21} {Stroke: 0.1% vs. 0.5% for TRI vs. TFI, OR 0.39 (95% CI 0.09-1.75), p=0.22}. When the three individual hard outcomes were combined, there was a marginal trend toward lower composite event rates in the transradial group {2.5% vs. 3.8%, (95% CI 0.49-1.01), p=0.058}.6) The large cohort data from 593,094 procedures in the National Cardiovascular Data Registry (606 sites; 2004 to 2007) were compared to the procedural success rate between TFI and TRI. Although the proportion of TRI procedures are rare {1.32% (7,804 cases) of total procedure}, TRI was associated with a similar rate of procedural success {adjusted OR: 1.02 (95% CI: 0.93-1.12)} compared with TFI.7) In a recent meta-analysis of acute MI performed primary PCI (n=3,324), there was significant reductions in the composite of death, MI, and stroke {3.65% vs. 6.55%, for TRI vs. TFI, OR 0.56 (95% CI 0.39-0.79)}.8)
Therefore, considerable evidence suggested that TRI has a similar procedural success and trend for reduction in ischemic events in coronary intervention compared with those of TFI.
Post-PCI bleeding complications are most commonly related to vascular access of the femoral artery. Femoral bleeding complications have traditionally been considered benign. However, the Mayo clinic data of 17,901 patients who underwent PCI between 1995 and 2006 found that major femoral bleeding complications were associated with increased 30-day mortality with a adjusted hazard ratio of 9.96 (95% CI: 6.94-14.3).4) Similar findings of a national cohort study showed that access site hematoma requiring transfusion independently associated with in-hospital mortality (OR 3.59, 95% CI 1.66-7.77) and 1-year death (OR 1.65, 95% CI 1.01-2.70).5) Currently, available data suggests that access site complications are not a benign complication of PCI, but a very important predictor of adverse procedural success and patient outcome.
A definite advantage of the transradial approach is the reduction in access site related complications such as access site bleeding, pseudoaneurysm, and arteriovenous fistula.1)7)8) In the meta-analysis of 23 randomized trials, radial access reduc-ed the OR of major bleeding by 73% in patients undergoing coronary angiography or intervention compared to femoral access.6) These benefits can be explained by the fact that the radial artery runs superficially and hemostasis can be easily achieved by manual compression or pressure bandage. In addition, no major nerves or veins were located near the radial artery, minimizing the risk of injury of these structures.
Some studies have shown that TRI could reduce both the bleeding complication and clinical outcomes. In the Mortality benefit Of Reduced Transfusion after percutaneous coronary intervention via the Arm or Leg (M.O.R.T.A.L) study from 38,872 PCI patients (radial approach, 20.5%), TRI was associated with a significant decrease in bleeding complications {OR 0.59 (95% CI 0.48 to 0.73)}, 30-day mortality {OR 0.71 (95% CI 0.61-0.82)} and 1-year mortality {OR 0.83 (95% CI 0.71-0.98)}, compared with those of TFI.9) In the meta-analysis with ST elevation MI, TRI reduces the risk of peri-procedural major bleeding {OR 0.30 (95% CI 0.16-0.55)}, and mortality {OR 0.54 (95% CI 0.33-0.86)} in the primary PCI setting.8) However, clinical evidence is limited because most of the study was observational or the data was obtained from an experienced operator. Therefore, large randomized clinical trials (RCT) are necessary to determine the benefit of TRI from access site bleeding to mortality and several large scale RCTs are ongoing (ClinicalTrials.gov Identifier; NCT01014273).
Another advantage of TRI is that it leads to early ambulation and may result in reduced hospital stay and decreased cost.10) Therefore, experienced centers of TRI establish an outpatient PCI unit, leading to increased turnover of PCI and diagnostic angiography cases and reduced cost for hospital stay.10)11) Many interventional cardiologists are becoming increasingly interested in TRI due to rare access site hematoma, patient convenience, and shortened hospitalization periods.
Although less procedural complications leads to an improvement of procedural outcomes, there are also downsides to the transradial approach. The transradial approach was associated with a significantly longer procedural time (mean difference: 3.1 minutes in meta-analysis) and fluoroscopic time (mean difference: 0.1 minutes in meta-analysis).6) However, there was significant heterogeneity (p<0.001, i2=87%) with a large difference in procedural time in studies performed by non-TRI experts {4.8 minutes (95% CI 3.7-5.8 minutes)} compared to TRI experts {1.7 minutes (95% CI 0.7-2.6 minutes)} suggesting that a significant learning curve is present.6)
A major problem in the early period may be access failure leading to crossover to another puncture site. In a recent meta-analysis, the rate of access site crossover was significantly higher with the TRI (5.9%, 150 of 2,542 patients) compared with the TFI (1.4%, 34 of 2,460 patients) {OR 3.82 (95% CI 2.83-5.15), p<0.001}.6) However, the OR for access site crossover decreased significantly from the earlier period of radial access to the modern period, suggesting that improvements in device technology and increase in expertise have narrowed the gap.
Another technical issue is related to the size of the guide catheter because the diameter of the radial artery is smaller than that of the femoral artery. In a meta-analysis, there was a slight insignificant trend towards higher failure rate of crossing lesion with wire, balloon, or stent with the transradial approach {OR 1.31 (95% CI 0.87-1.96), p=0.20}. However, in the studies performed by radial experts, the rates were similar between TRI and TFI {OR: 1.18 (95% CI 0.77-1.81), p=0.44}, again suggesting the presence of a learning curve.6) Overcoming the learning curve is usually dependant on TRI cases and an annual procedural volume >80 transradial cases are significant reductions in access failure, sheath insertion time, and overall procedural time in an observation study.12) With the development of dedicated catheters such as the sheathless system for TRI and with increased experience, there seem to be similar in access failure and procedure times between TRI and TFI.
In summary, TRI is associated with comparable procedural success and efficacy to TFI and reduces access site related complications and has made early ambulation possible, which significantly increases patient comfort and reduced hospital stay and cost. Therefore, TRI is a very attractive technique to apply in daily practice as a default route.
The indications of transradial PCI are expanding complex coronary lesions {e.g., chronic total occlusion (CTO),13)14) primary PCI,8)15)16) and bifurcation lesion17)} and non-coronary angioplasty (e.g., renal artery stenosis18) and peripheral artery disease19)) due to the miniaturization of devices used, improvement of devices, and accumulated experience with transradial PCI.
Although TRI has many advantages over TFI during interventional procedures, <10% of cath. labs use TRI as a default route.7)20) There are some obstacles to overcome the learning curve related to the radial artery access, selection of guide catheter and complex lesion angioplasty
Puncture failure is the first obstacle during the early learning period of TRI because radial artery is small (less than 4 mm) and prone to arterial spasm. In our experience, the wrist pain at the puncture site is an important factor leading to radial spasm and puncture failure. Premedication should be given, such as a light sedative or local anesthetic cream. Eutectic mixture of local anesthetic (EMLA) cream, composed of lidocaine 2.5% and prilocaine 2.5%, is known to be an effective topical anesthetic agent. It is used for a variety of painful cutaneous procedures on intact skin, including phlebotomy, intravenous catheterization, arterial cannulation and lumbar puncture.21) In our data, EMLA cream was able to reduce the pain (about half) during transradial cannulation and reducing the rate of puncture failure (Fig. 1).22) After sheath insertion, spasmolytic cocktails (various combinations of nitrate, xylocaine and verapamil) have been proposed to reduce the radial artery spasm during the procedures.
Variations and tortuosity of the radial artery have been observed during human dissections and on angiographic images. In routine clinical practice, variations of these vessels are one of the main reasons for technical difficulty and failure in TRI. In our data,23) tortuosity of radial and brachial artery was found in 4.2% (67 cases of 1,191 cases) and more frequently in elderly persons. The most common forms of tortuosity were S-shape in 21 cases, omega-shape in 21 cases and alpha-shape (radial artery loop) in 6 cases. In addition, the most common site of radial artery tortuosity was proximal third of antecubital fossa. Prolonged procedure times and/or cross-over to other routes were related with tortuosity of the radial artery (especially, omega and alpha-shape). However, the overall incidence of cross-over due to severe tortuosity of radial and brachial artery is rare (less than 1%) in our study.23)
Local vascular complications of TRI are rare compared to the femoral approach. The most frequent complication during TRI is the radial artery spasm as previously described. The other rare complications are severe hematoma or compartment syndrome due to small branch perforation of conduit artery and bleeding,24) radial artery occlusion,25) AV fistula (very rare), and causalgia caused by nerve injury during arterial puncture. Radial artery occlusion accounts for about 1% of transradial procedures. Most patients with radial artery occlusion are asymptomatic due to dual supply of the ulnar-radial artery. The use of an adequate amount of heparin is important in the prevention of radial artery occlusion after the procedure. The use of a standard 5,000 IU (relatively high dosage of transradial diagnostic procedure) or 50 U/kg of heparin is recommended.26)
A-V fistula is an extremely (<0.1%) rare situation that can arise after transradial procedures. Usually, they are asymptomatic with incidental accounts of bruit of puncture site. Radio-ulnar fistula can infrequently be corrected by surgery.
Severe bleeding in the forearm or compartment syndrome is a rare condition (<0.4%) after TRI procedures. The patient has symptoms of pain, pallor, painful stretching of muscle, paresthesia, and pulseless of forearms. Forearm bleeding can usually be observed after compression and rarely needs surgical intervention (surgical fasciotomy of forearm) due to ongoing ischemic changes of the forearm. Therefore, it is critical to immediately detect swellings of the access site or the path of catheter which are easily controlled with haemostatic compression.
For selecting the vascular access site, most interventional cardiologists are familiar with doing the procedure at the right side of the patient since the right radial approach is more convenient for manipulating the devices, including the guide catheter. Therefore, almost 90% of interventional cardiologists select the right radial artery as a first access route when performing transradial procedures in international survey data.20)
The only difference in performing the left radial access is that because the operator's standing position is apart from the left arm, the operator must bend over the patient, which may be inconvenient to the operator (Fig. 2). However, there are several advantages in selecting the left radial approach in terms of patient comfort and the effectiveness of the procedures. First, the left radial approach potentially increases patient comfort because most patients are right handed. The patients are free to use his/her right hand immediately after the procedure. Second, a left internal mammary angiogram can easily be performed in the case of a coronary artery bypass graft candidate. The advantages or disadvantages of right or left radial artery access are summarized in Table 1.
Our institute retrospectively evaluated the procedural success rate, crossover rate, puncture time, total procedural duration, fluoroscopy time, amount of contrast agent used and the local vascular complications in 711 cases via the left radial approach (Lt. group) and in 614 cases via the right radial approach (Rt. group).27) The puncture time, amount of contrast agent used, choice of the guide catheter and local vascular complications were similar for the two groups. There was no difference in the procedural success rate (Lt. group; 96.2% vs. Rt. group; 96.4%, p=not significant). However, there was a tendency towards a higher success rate via radial access for the Lt. group than for the Rt. group (Lt. group; 93.5% vs. Rt. group; 91.9%, p=0.056). The crossover rate was lower for the Lt. group than for the Rt. group (2.7% vs. 4.6%, respectively; p=0.04). The total procedural time (30.7±17.6 minutes vs. 32.3±15.4 minutes, respectively; p=0.03) and fluoroscopy time (13.9±7.9 minutes vs. 16.9±12.6 minutes, respectively; p<0.01) were shorter in the Lt. group. The number of guide catheters used was higher in the Rt. group compared to the Lt. group (1.21±0.48 vs. 1.08±0.33, respectively, p=0.04). Our data suggested that the left radial approach may provide increased procedural efficacy during transradial procedures.
Similar findings published in a randomized control trial (TALENT study) demonstrated that the left radial approach for TRI is associated with lower fluoroscopy time and radiation dose adsorbed by patients compared with the right radial approach, particularly in older patients.28) At present, the choice of the right or left radial artery as an entry site depends largely on operator preference. However, in case of higher frequency of right subclavian artery tortuosity (age >65, female, and short status <160 cm),29) the left radial approach might be better that the wight radial approach.
Diagnostic and PCI catheters for transradial approach are similar to those used for transfemoral approach at present. Recent advances in interventional device technology have provided miniaturization of the guiding catheter (GC). With the recent availability of 5 Fr. GC, dedicated shape GC and sheathless GC, the usefulness and indication of TRI will be expanded.
In the international survey data (75 countries) for TRI, a large majority of operators prefer to use 6 Fr GC. Only approximately 10% of operators use 5 Fr. GCs for TRI of right coronary artery, and <10% use 5-Fr. GCs for TRI of left coronary artery.20)
The selection of GC shapes depends on the site of coronary lesion in the survey data. For left coronary artery lesions, operators routinely use standard extra back-up (EBU) GCs. The most popular GC is EBU catheter (30% of all cases) in the international survey data.20) Amplatz, Kimny or Multipurpose GCs are also used for left coronary artery lesions. Interestingly, a significant number of operators still use less supportable GCs, such as Judkins left in 22.5% for left anterior descending artery lesions. For the right coronary lesions, the most popular GC shape is the Judkins right in 70.2% of cases. Amplatz left GC provides backup support for right coronary artery intervention. Therefore, Amplatz left GC can be used in the concomitant procedure in both coronary interventions.20)
Recent advancements in the technology of manufacturing TRI devices have now enabled us to use 5 Fr GC. Dahm et al.30) performed a randomized comparison of 6 Fr and 5 Fr GC by TRI to investigate procedural and clinical success rates and the rate of vascular access complications. TRI with 5 Fr GC shows a trend in favor of a higher procedural success rate and a lower rate of vascular access complications compared to 6 Fr GC, and these results were particularly marked in the subgroup of patients with small radial artery diameters.
Recently, the sheathless GC system (SheathLess Eaucath, Asahi Intecc®, Japan) consists of a hydrophilic GC and a central dilator. This system can be used to insert the GC into the radial artery without the use of an introducer sheath. The outer diameter of the 6.5 Fr sheathless GC (2.16 mm) is smaller than a 5 Fr introducer sheath (2.29 mm). The outer diameter of the 7.5 Fr sheathless GC (2.49 mm) is also less than that of 6 Fr introducer sheath (2.62 mm) (Fig. 3). With these features, the sheathless GC system enables performance of TRI even in patients with a small radial artery diameter.31) Furthermore, the hydrophilic coating that is present along the entire length of the GC may be helpful in reducing wrist pain due to arterial spasm. Mamas et al.32) and Liang et al.33) reported on successful use of a sheathless GC in complex interventions with large bore catheters including rotablation, crush stent bifurcation lesions, thrombectomy devices and simultaneous kissing stenting.
With the advancement of devices such as 5 Fr GC or sheathless GC system, TRI will expand the indication of various coronary lesion subsets and bulky device procedures with patient comfort, even for those with small radial artery size or spastic radial artery.
The indications for TRI are expanding due to an accumulation of experience with TRI and miniaturization of PCI devices. TRI interventionists could be feasible to a complex lesion subset (e.g., CTO and bifurcation lesion PCI) and unstable patient's haemodynamics (e.g., primary PCI and acute coronary syndrome). Although there is limited data for TRI of a complex lesion subset with regards to efficacy and the safety, recent data demonstrates similar procedural results and reduced access site complications compare with TFI.13-17)
In the era of potent antiplatelets and anticoagulants to reduce the incidence of ischemic events, primary PCI should be associated with high access site bleeding complication in the usual transfemoral procedure.34) Recent evidence showed that the burden of bleeding complications after primary PCI is closely related to the in-hospital morbidity and long term survival.8)35) Therefore, there are definite advantages of TRI in acute MI if primary PCI via radial artery can be performed with equal feasibility as TFI.
TEMPURA study showed that the success rate and major adverse cardiac events of transradial primary PCI were similar to transfemoral primary PCI, and hemorrhagic complications of transradial primary PCI including access site complications were decreased.15) In our data from 352 consecutive primary PCI cases,16) vascular access time was similar (3.8±3.5 minutes in TFI and 3.6±3.1 minutes in TRI) and cath. room to reperfusion time was 25±11 minutes in TRI and 26±13 minutes in TRI. Procedural success rate was 89% in TFI group and 88% in TRI group. Crossover occurred in 9 cases (4%) due to approaching vessel tortuosity in the TRI group. Major access site complication occurred in 7 cases (5%) in the TFI group and no complication in the TRI group (p<0.001). Although radial occlusion occurred in 5 cases of the TRI group, there was no evidence of hand ischemia. The total hospital stay was significantly shorter in TRI than TFI group. These data show transradial primary PCI is an effective and safe route in acute MI settings.
Recent meta-analysis (20 study involving 3,324 patients) confirms these findings.8) TRI reduced major bleeding compared to transfemoral PCI and significant reductions were found in the composite of death, MI, or stroke (p=0.001). Mortality showed a significant trend towards benefit in the case of transradial primary PCI {2.04% vs. 3.06%, OR 0.54 (95% CI 0.33-0.86), p=0.01}.
Collectively, transradial primary PCI reduces the risk of peri-procedural major bleeding and major adverse events with similar procedural success in the ST elevation acute MI settings.
There is limited data available for comparing TRI and TFI in treatment of CTO. When the radial approach for CTO intervention is attempted, availability of sufficient guiding support becomes a major concern since it is generally not feasible to use a GC larger than 7 Fr. Accordingly, the transfemoral approach is often preferred over transradial PCI for CTO because 7 or 8 Fr GCs may be used to obtain greater backup support, as compared to the 6 Fr GCs frequently used in the transradial approach.
However, several studies reported a high success rate for the transradial approach to CTO with acceptable crossover rate to femoral artery (3.3-6%).13)14) Saito et al.36) reported that the success rate was 67% in phase 1 and this improved to 81% in phase 2 (late phase). During phase 2, the success rate was higher for patients who were treated with TRI rather than with TFI (89% vs. 64%; p=0.008) which may indicate that CTO intervention is highly dependent on the experience and technique of the operators, regardless of access site. Rathore et al.14) examined 468 patients who underwent CTO PCI and compared the TRI (318 patients) and TFI (150 patients). The procedural success rate was similar in both groups (82% radial and 86% femoral, p=0.28). Total fluoroscopy time, total procedure time, and total contrast volume were also similar in TFI and TRI. The incidence of death or MI remained low in both groups, but, not surprisingly, the rate of access site complications (3.5% of TRI vs. 11.3% of TFI) and hematoma >5 cm (0% of TRI vs. 2.6% of TFI) was significantly lower in the radial group (p<0.001).
Collectively, the radial artery is a feasible vascular access route in coronary interventions for CTO with less access site complication and comparable procedural success as compared with TFI.
Many interventionists prefer the TFI for bifurcation lesions because coronary bifurcation lesions usually need the larger GCs which accommodate two stents or two balloons. Recent advancement and miniaturization of interventional devices enables us to perform bifurcation stenting with 6 Fr large-lumen GCs or sheathless GCs in case of small radial artery size. To date, there are limited data sets on TRI for bifurcation lesions.
In an observational study comparing immediate and 7-month follow-up clinical outcomes between TRI (n=60 patients) and TFI (n=74 patients) for true bifurcated lesions, there was no significant difference in immediate and long-term clinical outcomes.17)
Recently, the 7.5 Fr sheathless GC overcomes the lesions requiring simultaneous kissing stent implantation by transradial approaches. This sheathless system provides a large operating diameter and small outer catheter size compared to a conventional 6 Fr radial artery sheath introducer.33) Collectively, TRI of bifurcation lesion might be feasible, more effective and safer than TFI.
TRI was originally considered an alternative vascular route. However, due to technical improvements, enhanced device technology, and increased operator experience, TRI may become a default vascular access route currently for diagnostic coronary angiography, elective coronary angioplasty, primary PCI in acute MI and complex lesion PCI including CTO and bifurcating lesion.
Figures and Tables
Fig. 2
Procedural position of the right and left radial approaches for coronary interventions. A: left radial approach. B: right radial approach.
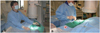
Fig. 3
Comparison of outer diameter (OD) between introducer sheath and sheathless guiding catheters (Adapted from reference 31 with authors' permission).
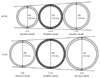
Table 1
Advantages and disadvantages of right vs. left radial artery approach

*Higher frequency of severe subclavian artery tortuosity29): Old age/Female/HTN/Small height/High body mass index
References
1. Agostoni P, Biondi-Zoccai GG, de Benedictis ML, et al. Radial versus femoral approach for percutaneous coronary diagnostic and interventional procedures: systematic overview and meta-analysis of randomized trials. J Am Coll Cardiol. 2004. 44:349–356.
2. Yoon J, Lee SH, Lee HH, et al. Usefulness of trans-radial coronary angiography in Wonju. Korean Circ J. 1998. 28:1670–1676.
3. Yoon J, Lee SH, Kim JY, et al. The experience of trans-radial coronary intervention in Wonju. Korean Circ J. 1998. 28:1443–1451.
4. Doyle BJ, Ting HH, Bell MR, et al. Major femoral bleeding complications after percutaneous coronary intervention: incidence, predictors, and impact on long-term survival among 17,901 patients treated at the Mayo Clinic from 1994 to 2005. JACC Cardiovasc Interv. 2008. 1:202–209.
5. Yatskar L, Selzer F, Feit F, et al. Access site hematoma requiring blood transfusion predicts mortality in patients undergoing percutaneous coronary intervention: data from the National Heart, Lung, and Blood Institute Dynamic Registry. Catheter Cardiovasc Interv. 2007. 69:961–966.
6. Jolly S, Amlani S, Hamon M, Yusuf S, Mehta SR. Radial versus femoral access for coronary angiography or intervention and the impact on major bleeding and ischemic events: a systematic review and meta-analysis of randomized trials. Am Heart J. 2009. 157:132–140.
7. Rao SV, Ou FS, Wang TY, et al. Trends in the prevalence and outcomes of radial and femoral approaches to percutaneous coronary intervention: a report from the National Cardiovascular Data Registry. JACC Cardiovasc Interv. 2008. 1:379–386.
8. Vorobcsuk A, Kónyi A, Aradi D, et al. Transradial versus transfemoral percutaneous coronary intervention in acute myocardial infarction: systematic overview and meta-analysis. Am Heart J. 2009. 158:814–821.
9. Chase AJ, Fretz EB, Warburton WP, et al. Association of the arterial access site at angioplasty with transfusion and mortality: the M.O.R.T.A.L study (Mortality benefit Of Reduced Transfusion after percutaneous coronary intervention via the Arm or Leg). Heart. 2008. 94:1019–1025.
10. Cooper CJ, El-Shiekh RA, Cohen DJ, et al. Effect of transradial access on quality of life and cost of cardiac catheterization: a randomized comparison. Am Heart J. 1999. 138:430–436.
11. Amoroso G, Laarman GJ, Kiemeneij F. Overview of the transradial approach in percutaneous coronary intervention. J Cardiovasc Med (Hagerstown). 2007. 8:230–237.
12. Spaulding C, Lefevre T, Funck F, et al. Left radial approach for coronary angiography: results of a prospective study. Cathet Cardiovasc Diagn. 1996. 39:365–370.
13. Kim JY, Lee SH, Choe HM, Yoo BS, Yoon J, Choe KH. The feasibility of percutaneous transradial coronary intervention for chronic total occlusion. Yonsei Med J. 2006. 47:680–687.
14. Rathore S, Hakeem A, Pauriah M, Roberts E, Beaumont A, Morris JL. A comparison of the transradial and the transfemoral approach in chronic total occlusion percutaneous coronary intervention. Catheter Cardiovasc Interv. 2009. 73:883–887.
15. Saito S, Tanaka S, Hiroe Y, et al. Comparative study on transradial approach vs. transfemoral approach in primary stent implantation for patients with acute myocardial infarction: results of the test for myocardial infarction by prospective unicenter randomization for access sites (TEMPURA) trial. Catheter Cardiovasc Interv. 2003. 59:26–33.
16. Kim JY, Yoon J, Jung HS, et al. Feasibility of the radial artery as a vascular access route in performing primary percutaneous coronary intervention. Yonsei Med J. 2005. 46:503–510.
17. Yang YJ, Xu B, Chen JL, et al. Comparison of immediate and follow up results between transradial and transfemoral approach for percutaneous coroanry intervention in true bifurcational lesions. Chin Med J. 2007. 120:539–544.
18. Kessel DO, Robertson I, Taylor EJ, Patel JV. Renal stenting from the radial artery: a novel approach. Cardiovasc Intervent Radiol. 2003. 26:146–149.
19. Sanghvi K, Kurian D, Coppola J. Transradial intervention of iliac and superficial femoral artery disease is feasible. J Interv Cardiol. 2008. 21:385–387.
20. Bertrand OF, Rao SV, Pancholy S, et al. Transradial approach for coronary angiography and interventions: results of the first international transradial practice survey. JACC Cardiovasc Interv. 2010. 3:1022–1031.
21. Gajraj NM, Pennant JH, Watcha MF. Eutectic mixture of local analgesics (EMLA) cream. Anesth Analg. 1994. 78:574–583.
22. Kim JY, Yoon J, Yoo BS, Lee SH, Choe KH. The effect of a eutectic mixture of local anesthetic cream on wrist pain during transradial coronary procedures. J Invasive Cardiol. 2007. 19:6–9.
23. Yoo BS, Yoon J, Ko JY, et al. Anatomical consideration of the radial artery for transradial coronary procedures: arterial diameter, branching anomaly and vessel tortuosity. Int J Cardiol. 2005. 101:421–427.
24. Lin YJ, Chu CC, Tsai CW. Acute compartment syndrome after transradial coronary angioplasty. Int J Cardiol. 2004. 97:311.
25. Stella PR, Kiemeneij F, Laarman GJ, Odekerken D, Slagboom T, van der Wieken R. Incidence and outcome of radial artery occlusion following transradial artery coronary angioplasty. Cathet Cardiovasc Diagn. 1997. 40:156–158.
26. Pancholy SB. Comparison of the effect of intra-arterial versus intravenous heparin on radial artery occlusion after transradial catheterization. Am J Cardiol. 2009. 104:1083–1085.
27. Kim JY, Yoon J, Jung IH, et al. Transradial coronary intervention: comparison of the left and right radial artery approach. Korean Circ J. 2006. 36:780–785.
28. Sciahbasi A, Romagnoli E, Burzotta F, et al. Transradial approach (left vs right) and procedural times during percutaneous coronary procedures: TALENT study. Am Heart J. 2011. 161:172–179.
29. Cha KS, Kim MH, Kim HJ. Prevalence and clinical predictors of severe tortuosity of right subclavian artery in patients undergoing transradial coronary angiography. Am J Cardiol. 2003. 92:1220–1222.
30. Dahm JB, Vogelgesang D, Hummel A, Stuudt A, Volzke H, Felix SB. A randomized trial of 5 vs 6 French transradial percutaneous coronary interventions. Catheter Cardiovasc Interv. 2002. 57:172–176.
31. Youn YJ, Yoon J, Sung JK, Kim JY. Feasibility of transradial coronary intervention using the sheathless guiding catheter in patients with small radial artery. Korean Circ J. 2011. In press.
32. Mamas MA, Fath-Ordoubadi F, Fraser DG. Atraumatic complex transradial intervention using large bore sheathless guide catheter. Catheter Cardiovasc Interv. 2008. 72:357–364.
33. Liang M, Puri A, Linder R. Transradial simultaneous kissing stenting (SKS) with sheathless access. Catheter Cardiovasc Interv. 2010. 75:222–224.
34. Nikolsky E, Mehran R, Dangas G, et al. Development and validation of a prognostic risk score for major bleeding in patients undergoing percutaneous coronary intervention via the femoral approach. Eur Heart J. 2007. 28:1936–1945.
35. Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KA, Yusuf S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation. 2006. 114:774–782.
36. Saito S, Tanaka S, Hiroe Y, et al. Angioplasty for chronic total occlusion by using tapered-tip guidewires. Catheter Cardiovasc Interv. 2003. 59:305–311.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download