Abstract
Primary cardiac lymphomas (PCL) are extremely rare. Clinical manifestations may be variable and are attributed to location. Here, we report on a case of PCL presenting with atrioventricular (AV) block. A 55 year-old male had experienced chest discomfort with unexplained dyspnea and night sweating. His initial electrocardiogram (ECG) revealed a first degree AV block. Along with worsening chest discomfort and dyspnea, his ECG changed to show second degree AV block (Mobitz type I). Computed tomography (CT) scan showed a cardiac mass (about 7 cm) and biopsy was performed. Pathologic finding confirmed diffuse large B-cell lymphoma. The patient was treated with multi-drug combination chemotherapy (R-CHOP: Rituximab, cyclophoshamide, anthracycline, vincristine, and prednisone). After treatment, ECG changed to show normal sinus rhythm with complete remission on follow-up CT scan.
Primary cardiac lymphomas (PCL) are extremely rare, accounting for only 2% of primary cardiac tumors and 0.5% of extranodal lymphomas. Clinical manifestations may vary and are attributed to location, and presence of congestive heart failure, pericardial effusion, or arrhythmia. Here we report a case of PCL presenting with atrioventricular (AV) block.
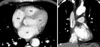
A 55 year-old man with no previous history of cardiac disease and well controlled hypertension visited the outpatient cardiology department. The patient had experienced discomfort in his chest with unexplained dyspnea for 4 days. He complained of severe night sweats as well, but could not recall the exact time when symptoms started. He had smoked a pack a day for the past 30 years. Findings from physical examination were not specific; no palpable lymph nodes, palpable liver, or spleen were observed. Initial lab results showed no remarkable findings, with the exception of mild pro Brain Natriuretic Peptide (BNP) elevation to 568.4 pg/mL. The patient's initial electrocardiogram (ECG) revealed first degree AV block (Fig. 1). Chest radiography was close to normal. A well delineated lesion adjacent to the ascending aorta was observed in transthoracic echocardiography (TTE). Consequently, thoracic aorta CT was performed; a multi-lobulating, low density mass (about 7 cm) with an irregular shape was observed in the right atrium (RA) and extended to the periaortic space of the ascending aorta and left atrium (Fig. 2). The lesion adjacent to the ascending aorta in TTE was revealed as a cardiac mass by CT scan. The patient was referred to the hemato-oncology department, and scheduled to undergo biopsy of the cardiac mass within a few days at the department of chest surgery.
However, before the scheduled appointment for biopsy, the patient was rushed to the emergency room for worsening chest discomfort, dyspnea, and palpitation. Mobitz type I in second degree AV block was detected on ECG while the patient was complaining of symptoms (Fig. 3A). The patient underwent 24 hour holter monitoring. Although there was no direct clinical correlation with AV block, 2:1 AV block was found in the readings (Fig. 3B). Mediastinotomy via right parasternal incision was performed, followed by tumor biopsy. Surgical findings showed that the mass was not engaging with the pericardium and that it surrounded the RA and aorta, as in the CT reading. Partial invasion of the mass into the myocardium was observed, but in general, the mass was not engaged. H&E staining revealed large lymphocytes with high mitotic activity showing diffuse infiltration. In immunochemical staining, CD 20 (the B cell marker) showed a positive result, while CD 3 (the T cell marker) showed a negative result (Fig. 4). Pathologic finding confirmed diffuse large B-cell lymphoma. The lymphoma staging work-up included abdominal pelvic CT scan, bone marrow biopsy, and otolaryngologic exam. Beyond the cardiac lesion observed during the extensive evaluation described above, there was no evidence of lymphoma involvement. The patient received a final diagnosis of primary cardiac diffuse large B-cell lymphoma. The patient was treated with 6 cycles of combination chemotherapy (R-CHOP: Rituximab, cyclophoshamide, anthracycline, vincristine, and prednisone). After 3 cycles of treatment, his ECG was converted to normal sinus rhythm (Fig. 5), and follow-up CT scan showed that the cardiac mass had almost completely disappeared (Fig. 6).
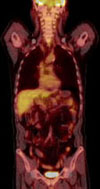
After 6 cycles of treatment, a positron emission tomography (PET) CT scan was performed from cerebellum to pelvis. PET-CT scan inclusive of the heart and other sites found no evidence of hypermetabolic lesion, an indication of malignancy (Fig. 7). The patient went into a state of complete remission and was followed up at our outpatient hemato-oncology department.
PCL is extremely rare, accounting for only 2% of primary cardiac tumors1) and 0.5% of extranodal lymphomas.2) About 100 cases have been described in the literature, mostly as case reports or small case series.3) Only 6 cases have been reported in Korea.4-8) However, secondary cardiac involvement of malignant lymphoma is relatively common and was found in up to 25% of autopsy cases.9)
In 1977, McAllister and Fenoglio2) defined PCL as an extranodal lymphoma involving only the heart and/or pericardium. Cairns et al.10) suggested that PCL could also present elsewhere as small secondary lesions, with the vast bulk of the tumor arising in the heart. Currently, the accepted definition of PCL is a lymphoma presenting as cardiac disease, particularly if the bulk of the tumor is intrapericardial.11)
Pathognomonic clinical presentation has not been described, and is generally related to sites of involvement in the heart. In the majority of cases, PCL was reported as arising in the right chambers of the heart; the atria are involved in most patients studied. Right-sided involvement predominates in primary cardiac lymphoma, in contrast to left-sided predominance in atrial myxoma, which is the major primary benign cardiac tumor.
The most common presentation is arrhythmia, followed by pericardial effusion, with tamponade, dyspnea, and cardiac failure. Unusual presentations, including superior vena cava syndrome, embolic stroke, or symptoms suggestive of gastrointestinal disease may occur.
The ECG was found not to be useful, and chest radiography tended to reveal only nonspecific findings, such as a cardiomegaly or pleural effusion. TTE identified an intracardiac mass in 60% of cases, and transoesphageal echocardiography (TEE) identified intracardiac masses in 97% of cases.12) Echocardiography represents the most sensitive noninvasive technique for identification of PCL, particularly the transesophageal approach. CT and cardiac magnetic resonance (MR) are the techniques most commonly utilized. Contrast enhanced CT and MR offer the advantages of better contrast resolution and simultaneous visualization of the great vessels, heart, pericardium, mediastinum, and lung. Furthermore, CT and MR may present information useful for differential diagnosis of tumors, depending on their CT density and/or MR signal intensity characteristics, particularly in cardiac lipoma, osteochondrosarcoma, and pericardial cysts.13) Usefulness of nuclear medicine techniques, such as PET CT, has also been demonstrated in identification of metastatic foci and in follow-up.
Definitive diagnosis of PCL is made on the basis of histology/cytology and is mandatory for appropriate therapy. If pericardial or pleural effusions are present, cytology is diagnostic in only two-thirds of cases.14) When cytology is not available, diagnosis of PCL is determined primarily by biopsy of cardiac tissue during explorative thoracotomy. To avoid thoracotomy, less invasive procedures, including mediastinoscopy, TEE-guided biopsy, thoracoscopic pericardial window, and endomyocardial transvenous biopsy are performed.
According to the World Health Organization classification, diffuse B-cell lymphoma, mainly of the large cell subtype, was observed in most cases.
Chemotherapy is the only effective treatment for PCL (radiotherapy does not seem to improve patient survival rate, and a radical surgical approach is not recommended). R-CHOP is the primary chemotherapy regimen. The first cycle of chemotherapy must be given with caution due to a high risk of cardiac rupture during rapid tumor regression.15)
In most patients, the RA is affected and infiltration within the conductive system can produce many types of arrhythmias: atrial fibrillation, AV block, sick sinus syndrome, ventricular tachycardia, and ventricular fibrillation.
However, the treatment of arrhythmia itself is not a difference. Occasionally, as in this case, the arrhythmia itself is improved after lymphoma treatment.
In this case, as shown on CT scan, lymphoma cells infiltrated into the AV node, leading to AV block. The patient's ECG returned to normal sinus rhythm after R-CHOP chemotherapy, proving that infiltration affected the conductive system. In the English literature, about 20 cases presenting with AV block have been described. Among those cases, a pacemaker was inserted in patients with high degree AV block.16-18) To the best of our knowledge, this case is the first to report on PCL with AV block in the Korean literature.
Figures and Tables
Fig. 2
Thoracic aorta CT demonstrating a multi-lobulating, low density mass (about 7 cm) with an irregular shape in the right atrium, extending to the periaortic space of the ascending aorta and left atrium (arrows). A: coronal view. B: sagittal view. LV: left ventricle, LA: left atrium, RV: right ventricle.
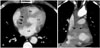
Fig. 3
ECG showing second degree AV block. A: Mobitz type I. B: 2 : 1 AV block. AV: atrioventricular, ECG: electrocardiogram.
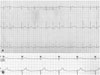
Fig. 4
Histologic findings. A: in H&E staining, large lymphocytes with high mitotic activity showed diffuse infiltration. In immunochemical staining. B: CD 20, the B cell marker, showed a positive result. C: CD 3, the T cell marker, showed a negative result.

References
1. Fernandes F, Soufen HN, Ianni BM, Arteaga E, Ramires FJ, Mady C. Primary neoplasms of the heart: clinical and histological presentation of 50 cases. Arq Bras Cardiol. 2001. 76:231–237.
2. McAllister HA, Fenoglio JJ. Tumors of the cardiovascular system. Atlas of Tumor Pathology. 1978. Washington. DC: Armed Forces Institute of Pathology;99–100. 2nd Series. Fascicle 15.
3. Nascimento AF, Winters GL, Pinkus GS. Primary cardiac lymphoma: clinical, histologic, immunophenotypic, and genotypic features of 5 cases of a rare disorder. Am J Surg Pathol. 2007. 31:1344–1350.
4. Chang HJ, Kang SM, Rim SJ, et al. A case report of primary cardiac lymphoma: diagnosis by transvenous biopsy. Korean Circ J. 1999. 29:828–832.
5. Kang SB, Jin SW, Lee EK, et al. A case of non-Hodgkin's lymphoma with massive involvement of the right atrium. Korean Circ J. 2000. 30:492–496.
6. Ryu SJ, Choi BW, Choe KO. CT and MR findings of primary cardiac lymphoma: report upon 2 cases and review. Yonsei Med J. 2001. 42:451–456.
7. Kim JY, Woo CM, Lee JY, et al. A case of primary cardiac non-Hodgkin's lymphoma. Korean Circ J. 2004. 34:808–812.
8. Choi WS, Han IY, Jun HJ, Lee YH, Hwang YH, Cho KH. Primary malignant cardiac lymphoma in right atrium: a case report. Korean J Thorac Cardiovasc Surg. 2008. 41:369–372.
9. Robert WC, Glancy DL, De Vita VT Jr. Heart in malignant lymphoma (Hodgkin's disease, lymphosarcoma, reticulum cell sarcoma and mycosis fungoides): a study of 196 autopsy cases. Am J Cardiol. 1968. 22:85–107.
10. Cairns P, Butany J, Fulop J, Rakowski H, Hassaram S. Cardiac presentation of non-Hodgkin's lymphoma. Arch Pathol Lab Med. 1987. 111:80–83.
11. Burke A, Virmani R. Tumors of the heart and great vessels. Atlas of Tumor Pathology. 1996. Washington, DC: Armed Forces Institute of Pathology;171–179. 3rd Series. Fascicle 16.
12. Faganello G, Belham M, Thaman R, Blundell J, Eller T, Wilde P. A case of primary cardiac lymphoma: analysis of the role of echocardiography in early diagnosis. Echocardiography. 2007. 24:889–892.
13. Araoz PA, Mulvagh SL, Tazelaar HD, Julsrud PR, Breen JF. CT and MR imaging of benign primary cardiac neoplasm with echocardiographic correlation. Radiographics. 2000. 20:1303–1319.
14. Ceresoli GL, Ferreri AJ, Bucci E, Ripa C, Ponzoni M, Villa E. Primary cardiac lymphoma in immunocompetent patients: diagnostic and therapeutic management. Cancer. 1997. 80:1497–1506.
15. Molajo AO, McWilliam L, Ward C, Rahman A. Cardiac lymphoma: an unusual case of myocardial perforation: clinical, echocardiographic, haemodynamic and pathological features. Eur Heart J. 1987. 8:549–552.
16. Nagano M, Uike N, Suzumiya J, et al. Successful treatment of a patient with cardiac lymphoma who presented with a complete atrioventricular block. Am J Hematol. 1998. 59:171–174.
17. Tai CJ, Chen PM, Wang WS, et al. Complete atrioventricular block as a major clinical presentation of the primary cardiac lymphoma: a case report. Jpn J Clin Oncol. 2001. 31:217–220.
18. Takenaka S, Mitsudo K, Inoue K, et al. Successful treatment of primary cardiac lymphoma with atrioventricular nodal block. Int Heart J. 2005. 46:927–931.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download