Abstract
As techniques and device technology have improved, the success rates and long patency of ilio-femoral occlusive disease have also improved. In the case of extensive iliac occlusive disease, however, wire passage and handling remain a challenge due to the relatively weak guiding catheter backup support with the contralateral femoral approach. There has been no report on methods to overcome this problem. We performed a successful percutaneous translunimal angioplasty for long ilio-femoral occlusive disease including the iliac ostium by a dual approach including simultaneous brachial and contralateral femoral arteries for subintimal angioplasty.
When performing a percutaneous transluminal angioplasty (PTA) for common iliac artery (CIA) occlusive lesions involving the ostium, we usually experience difficulty in passage or handling of the wire due to the relatively weak guiding catheter backup support with the contralateral femoral approach. Furthermore, in situations involving a very long ilio-femoral artery, it is very difficult to finalize the procedure because of practical limitations including a short balloon length and difficulty with wire manipulation. Here we present a dual approach including simultaneous brachial and contralateral femoral arteries for subintimal angioplasty in a patient with long ilio-femoral occlusive disease including the iliac ostium.
A 79-year-old man was admitted to our hospital due to necrosis of the left third toe, anterior tibial ulceration, and claudication (Fontaine IV). Symptoms developed one year ago, and the patient's clinical status has deteriorated over the past week. The patient was treated for pulmonary tuberculosis 20 years ago; however, his sputum acid-fast bacilli stain continues to be positive. He was a current smoker with a 20-pack-year history. The chest X-ray showed significant lung volume reduction due to tuberculosis related destruction. The echocardiography showed a left ventricular ejection fraction of 35% with global hypokinesia. The coronary angiography revealed insignificant stenosis. Computed tomography-angiography (CTA) of the lower extremities showed total occlusion from the left CIA ostium to the distal femoral artery with a reasonably collateralized circulation (Fig. 1A). The ankle-brachial index was zero for the left side, and 0.97 for the right side.
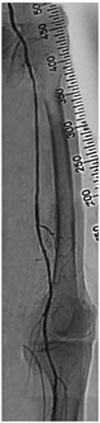
Because of the high risk of general anesthesia and patient refusal to undergo surgical revascularization, endovascular treatment was planned for the extensive iliofemoral occlusive disease. The antegrade approach from the contralateral common femoral artery was not possible due to the iliac ostium lesion, as shown in the CTA of the lower extremity. Lower limb angiography, therefore, was performed via the right brachial artery with a six French shuttle sheath. The angiography revealed total occlusion of left CIA and left common femoral artery with collateral flow that reconstructed the distal superficial femoral artery (SFA) (Fig. 2A). Using a 0.035 inch angled Terumo wire with a backup catheter (4F multipurpose catheter, Merit Medical, South Jordan, Utah, USA), successful wiring from the CIA to the external iliac artery was performed by subintimal wire tracking without significant dissection (Fig. 2B). The iliac artery lesion was predilated with a 6.0×40 mm balloon (10 atm, Powerflex, Cordis, Bridgewater, NJ, USA) (Fig. 2C). After predilation, subintimal dye staining was noted. A six French Balkin sheath was inserted through the contralateral femoral artery (Fig. 3A), and a 0.035 inch angled Terumo wire was successfully passed through the SFA by the transfemoral approach (Fig. 3B). A 10.0×60 mm peripheral self-expandable stent (Smart, Cordis) was deployed in the CIA to the external iliac artery (Fig. 3C). After post-dilatation with an 8.0×40 mm balloon (10 atm, Powerflex, Cordis) in the CIA to the external iliac artery, an 80% diffuse irregular stenosis at the proximal SFA was noted. Predilatation was performed with a 4.0×40 mm, and 5.0×40 mm (10 atm, Powerflex, Cordis) balloon; however, residual stenosis was present at about 60% or more. The pressure gradient (50 mmHg) remained at the femoral head area with dissection in spite of the additional ballooning using the 5.0×80 mm balloon (10 atm, Powerflex, Cordis). Then, a 7.0×40 mm self-expandable stent (Smart, Cordis) was deployed in the SFA and additional ballooning was performed using a 5.0×80 mm (10 atm, Powerflex, Cordis) for the CIA and SFA (Fig. 3D). The final angiography showed patent stents with good distal flow (Fig. 4), and the pressure gradient between the CIA and popliteal artery was less than 10 mmHg.
The follow up CTA showed no residual stenosis from the CIA to the distal femoral artery (Fig. 1B). The patient had no residual symptoms and the left ankle-brachial index was improved to 0.96 after the successful angioplasty.
The TransAtlantic InterSociety Consensus (TASC) statement in 1999 provided a framework for clinicians to use to assess the treatment of aortoiliac and femoral lesions in the peripheral vasculature by stratifying the lesion length and morphology.1) At the time of that publication, the available data suggested that most iliac occlusions should be treated with surgical revascularization. Most articles about bypass surgery were written in the 1970s and 1980s, before endovascular therapy began to replace open reconstruction.2) The endovascular treatment of ilio-femoral occlusive disease has evolved over the last two decades. As CTA imaging, balloon angioplasty, stent technology, and endovascular techniques have improved, success rates and long patency of ilio-femoral occlusive disease has also improved.
In patients with extensive iliac occlusive disease, open revascularization is the treatment of choice in current vascular surgical practice, according to the TASC statement.1) Surgical revascularization, however, also has the risks associated with general anesthesia, and cannot be performed in all patients. Recent reports have shown a decreasing number of patients undergoing aorto-bifemoral bypass.3)4) In addition, endovascular procedures are often used as first-line therapy for aortoiliac occlusive disease; this is because many believe that surgical options are available if the endovascular therapy is unsuccessful. PTA has the additional advantage in that it can be performed without hemodynamic changes. Kashyap et al.5) compared bypass surgery with PTA. In this report, there was no significant difference in the secondary patency rates, limb salvage, and long-term survival between the two groups. The patients undergoing PTA with TASC-D lesions tended to have better patency. Several studies have documented the outcomes of treatment of more complex iliac occlusions of the TASC-C and TASC-D. Several studies have shown that the primary patency ranged from 69% to 76% at 2 years, with the secondary patency rates of 85% to 95% at 2 years.6-9) Furthermore, the overall complication rates were reported to be low in a recent series (1.4-4.8%), likely because of improvements in technique and device technology.
The most common techniques used for recanalization of iliac ostium occlusive disease are usually performed with a retrograde approach from the ipsilateral common femoral artery or popliteal artery, an antegrade approach from the left brachial artery, or an antegrade approach from the contralateral CIA. In some cases, PTA has been performed successfully by using devices designed for coronary intervention.10) However, in situations involving a very long ilio-femoral artery, patients require multiple access sites for successful treatment, because of practical limitations including short balloon length. For a successful PTA, not only device technology but also specialized backup supports are needed. We performed a successful PTA in a patient with long ilio-femoral occlusive disease that included the iliac ostium by a dual approach. The PTA was carried out at the iliac ostial lesion via the antegrade approach from the right brachial artery. After recanalization of the iliac ostium, the ilio-femoral lesion was treated by the antegrade approach from the contralateral femoral artery. Using this dual approach, we could perform successful endovascular treatment by virtue of the good backup support.
Although this report relates to only one patient, in certain situations where both contralateral femoral and ipsilateral popliteal access is difficult because of concomitant vascular disease or variation in anatomy, the dual approach technique of simultaneous brachial and contralateral femoral artery access may broaden the endovascular therapeutic possibilities for treatment of long ilio-femoral occlusive disease including the iliac ostium.
In conclusion, the dual approach technique of simultaneous brachial and contralateral femoral artery access enabled treatment of total occlusive lesions associated with long ilio-femoral occlusive disease, including the iliac ostium, without significant complications. PTA should be considered as the first choice for a revascularization procedure; it is safe and effective for limb salvage in patients with critical limb ischemia.
Figures and Tables
Fig. 1
Computed tomography-angiography (CTA) of the lower extremity. (A) shows total occlusion from the left common iliac artery ostium to the distal femoral artery with a reasonably collateralized circulation; after percutaneous transluminal angioplasty, the follow-up CTA revealed no residual stenosis from the common iliac artery to the distal femoral artery as shown in (B).
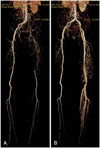
Fig. 2
Subintimal wiring for iliac occlusion through right brachial artery. Lower limb angiography via the right brachial artery revealed total occlusion of the left common iliac artery and left common femoral artery with collateral flow that reconstructed the distal superficial femoral artery as shown in (A). Using a 0.035 inch angled Terumo wire with a backup catheter; successful wiring from the common iliac artery to the external iliac artery was performed by subintimal wire tracking as shown in (B). After predilation (C), subintimal dye staining was noted as shown in (D).

Fig. 3
Subintimal wiring for superficial femoral artery occlusion via the contralateral femoral artery. A six French Balkin sheath was inserted through the contralateral femoral artery (A), and a 0.035 inch angled Terumo wire was successfully passed through the superficial femoral artery by the antegrade approach via the right femoral artery (B). A 10.0×60 mm peripheral self-expandable stent (Smart, Cordis) was deployed in the common iliac artery to the external iliac artery (C). Percutaneous transluminal angioplasty for the common iliac artery and superficial femoral artery was carried out (D).

References
1. Dormandy JA, Rutherford RB. Management of peripheral arterial disease (PAD): TransAtlantic InterSociety Consensus (TASC). J Vasc Surg. 2000. 31:S1–S296.
2. Rutherford RB. Options in the surgical management of aortoiliac occlusive disease: a changing perspective. Cardiovasc Surg. 1999. 7:5–12.
3. Whiteley MS, Ray-Chaudhuri SB, Galland RB. Changing patterns in aortoiliac reconstruction: a 7-year audit. Br J Surg. 1996. 83:1367–1369.
4. Upchurch GR, Dimick JB, Wainess RM, et al. Diffusion of new technology in health care: the case of aorto-iliac occlusive disease. Surgery. 2004. 136:812–818.
5. Kashyap VS, Pavkov ML, Bena JF, et al. The management of severe aortoiliac occlusive disease: endovascular therapy rivals open reconstruction. J Vasc Surg. 2008. 48:1451–1457.
6. Dyet JF, Gaines PA, Nicholson AA, et al. Treatment of chronic iliac artery occlusions by means of percutaneous endovascular stent placement. J Vasc Interv Radiol. 1997. 8:349–353.
7. Henry M, Amor M, Ethevenot G, Henry I, Mentre B, Tzvetanov K. Percutaneous endoluminal treatment of iliac occlusions: long term follow-up in 105 patients. J Endovasc Surg. 1998. 5:228–235.
8. Uher P, Nyman U, Lindh M, Lindblad B, Ivancev K. Long-term results for stenting chronic iliac artery occlusions. J Endovasc Ther. 2002. 9:67–75.
9. Carnevale FC, De Blas M, Merino S, Egana JM, Caldas JG. Percutaneous endovascular treatment of chronic iliac artery occlusion. Cardiovasc Intervent Radiol. 2004. 27:447–452.
10. Lee SH, Park HS, Jang SY, et al. A case of peripheral revascularization via the radial artery using devices designed for percutaneous coronary intervention. Korean Circ J. 2008. 38:671–673.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download