Abstract
Primary cardiac angiosarcoma is a very rare disease with a poor prognosis. We report a case of a patient with a primary cardiac angiosarcoma who presented with cardiac tamponade; the angiosarcoma was successfully resected surgically.
Primary cardiac tumors are rare. Twenty-five percent of primary cardiac tumors are malignant. The most common type of primary malignant cardiac tumor is an angiosarcoma, followed by leiomyosarcomas, rhabdomyosarcomas, and osteosarcomas.
The prognosis of primary malignant cardiac tumors remains poor. The prognosis of angiosarcomas is worse than that of other histologic types. High mortality rates of angiosarcomas are attributed to the rapid local relapse and high incidence of systemic metastasis.
We report a patient with a primary cardiac anigosarcoma who presented with a massive pericardial effusion and was treated surgically.
A 63-year-old man was admitted for chest discomfort, orthopnea, and dyspnea on exertion lasting 5 days. His initial vital signs included a blood pressure of 100/60 mmHg, a pulse rate of 80 beats/min, a respiratory rate of 20/min, and a body temperature of 36.6℃.
The jugular vein was engorged, but no hepatomegaly or audible cardiac murmurs were detected. The laboratory tests revealed the following: a hemoglobin, 11.3 g/dL; hematocrit, 35.3%; white blood cell (WBC) count, 9,550/mm3; platelet count, 254,000/mm3; blood urea nitrogen, 14 mg/dL; serum creatinine, 1.0 mg/dL; alanine aminotransferase, 58 IU/L; aspartate aminotransferase, 40 IU/L; total bilirubin, 0.9 mg/dL; glucose, 166 mg/dL; lactate dehydrogenase, 444 IU/L; protein, 6.4 g/dL; and albumin, 3.7 g/dL. Chest radiography showed an increased cardiac silhouette with a round, flask-like appearance (Fig. 1). Computed tomography and transthoracic echocardiography showed a large inhomogeneous and focally-enhancing mass in the right atrium, and a massive amount of pericardial and right pleural effusion (Fig. 2).
While in the emergency room, the blood pressure dropped to 80/54 mmHg and the pulse rate increased to 101 beats/min; the patient complained of severe dyspnea and chest pain, suggesting cardiac tamponade. An emergency pericardiocentesis and drainage was performed. The pericardial effusion was blood-like in color; an effusion analysis showed the following: hemoglobin, 11.9 g/dL; red blood cell (RBC) count, 2.95×106/mm3; WBC, 15,210/mm3 (lymphocytes, 68%; neutrophils, 23%), glucose, 109 mg/dL; lactate dehydrogenase, 591 IU/L; and protein, 5.6 g/dL. The initial drainage of the pericardial effusion was 800 mL. There were no malignant cells in the pericardial effusion cytology. The chest pain and dyspnea were improved immediately after the pericardiocentesis. Transesophageal echocardiography showed a 5.5×5 cm mass without invasion of the inferior and superior vena cava (Fig. 3).
On hospital day (HD) 5, surgery was performed. After the mass was resected, the right atrium was repaired with a bovine pericardial patch. The mass was close to the tricuspid valve (only 2 mm from the resection margin). On HD 7, the patient was transferred to the general ward and on HD 13 he was discharged. The patient was scheduled to receive adjuvant radiotherapy and chemotherapy.
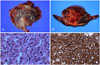
At the time of biopsy, a 6.8×6.5×2.5 cm gray-brown protruding endocardial mass was noted in the right atrial chamber. The pericardium was spared of malignant cells. The cut surface was diffusely hemorrhagic with a gray-white solid portion. The tumor was comprised of oval-to-spindle cells with intracytoplasmic RBCs. There were frequent mitoses (> 10/10 HPF). The tumor cells were positive for CD34 immunohistochemical staining with confirmed primary cardiac angiosarcoma (Fig. 4).
Primary cardiac tumors are very rare. Secondary or metastatic cardiac tumors are 20-40 times more common than primary cardiac tumors, such as lymphomas, leukemias, malignant melanomas, and lung and breast cancers. The incidence of primary cardiac tumors is approximately 0.02% based upon the data of 22 large autopsy series.1)2) Twenty-five percent of primary cardiac tumors are malignant.3)
Dyspnea on exertion is the most common symptom of primary cardiac sarcomas at the time of presentation (79%), followed by non-specific chest pain (38%), cough (21%), paroxysmal nocturnal dyspnea (12%), hemoptysis (12%), embolic events (9%), and fever (9%).4)
Several diagnostic tools for primary cardiac tumors are available. Echocardiography is a screening modality, showing tumor size, location, mobility, attachment, and transesophageal echocardiography better depicts the posterior wall of the left atrium, atrial septum, right heart, and descending aorta. Diagnostic sensitivities of transthoracic and transesophageal echocardiography are 93.3% and 96%, respectively.5) Cardiac computed tomography and magnetic resonance imaging can reveal infiltrative growth and the extracardiac extent of sarcomas, which are criteria that help distinguish benign from malignant lesions and assess respectability.6)7) Coronary arteriography may demonstrate tumor blood supply and distortion of coronary anatomy, but is not essential in diagnosis.8)
The currently established treatment of cardiac sarcomas is limited to surgical resection. Complete surgical resection is the most important prognostic factor.9) Other prognostic factors include left-sided lesion, absence of necroses or metastases, and low mitotic count. Age, gender, and histologic grade do not appear to affect the prognosis.4) Causes of death were metastasis or heart failure in two case reports.10)11)
Llombart-Cussac et al.12) reported that survival of patients with primary cardiac angiosarcomas who underwent complete resection is 22 months, while those who underwent incomplete resection is 7 months in. Alvarez et al.13) reported that the mean overall survival in patients with primary cardiac angiosarcomas was 5 months and on subgroup analysis, survival was 3-12 months in patients who underwent surgery plus adjuvant treatment, whereas 5 days to 7 months in those who underwent surgery only. However, subsequent studies have failed to show the survival benefit of adjuvant treatment.9)14) Heart and lung transplantation has been attempted, but limited experience resulted in poor outcome due to local recurrence and metastasis post-operatively.15)
This patient presented with a locally advanced tumor with cardiac tamponade without evidence of metastasis. Although the tumor was close to the tricuspid valve, it was totally resected with a narrow, but clear margin on microscopic exam. We expected a survival benefit for this patient from adjuvant treatment following complete surgical resection. On day 30 post-operatively, the patient was readmitted for concurrent chemo-radiotherapy. Radiotherapy was planned for a total dose of radiation of 45 Gray in 25 fractions; however, the patient refused further radiotherapy and chemotherapy after four cycles of radiotherapy. The patient has survived for 4 months after diagnosis. He is attending our hospital's outpatient department.
Figures and Tables
Fig. 1
Round, increased cardiac silhouette and right costophrenic angle blunting on chest radiography.
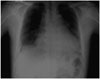
Fig. 2
Chest CT (A) and transthoracic echocardiography (B) shows a large and inhomogeneous, enhancing mass in the right atrium (arrow) and a massive amount of pericardial and right pleural effusion.
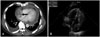
Fig. 3
Transesophageal echocardiography shows a primary cardiac mass without involvement of the inferior and superior vena cava. IVC: inferior vena cava, SVC: superior vena cava, RA: right atrium, LA: left atrium.
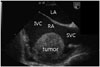
Fig. 4
A: a resected protruding mass (6.8×6.5×2.5 cm) into the right atrial chamber is present at the atrial wall. B: the cut surface is diffusely hemorrhagic with a gray-white fleshy solid portion. C: the tumor is composed of oval-to-spindle cells with intracytoplasmic red blood cells. There are frequent mitoses (> 10/10 HPF). D: the tumor cells are positive for CD34 immunohistochemical staining.
References
1. Reynen K. Frequency of primary tumors of the heart. Am J Cardiol. 1996. 77:107.
2. Hanfling SM. Metastatic cancer to the heart: review of the literature and report of 127 cases. Circulation. 1960. 22:474–483.
3. Burke AP, Cowan D, Virmani R. Primary sarcomas of the heart. Cancer. 1992. 69:387–395.
4. Simpson L, Kumar SK, Okuno SH, et al. Malignant primary cardiac tumor. Cancer. 2008. 112:2440–2446.
5. Meng Q, Lai H, Lima J, Tong W, Qian Y, Lai S. Echocardiographic and pathologic characteristics of primary cardiac tumors: a study of 149 cases. Int J Cardiol. 2002. 84:69–75.
6. Dawson WB, Mayo JR, Muller NL. Computed tomography of cardiac and pericardial tumors. Can Assoc Radiol J. 1990. 41:270–275.
7. Shin MS, Kirklin JK, Cain JB, Ho KJ. Primary angiosarcoma of the heart: CT characteristics. AJR Am J Roentgenol. 1987. 148:267–268.
8. Smith DN, Shaffer K, Patz EF. Imaging features of nonmyxomatous primary neoplasms of the heart and pericardium. Clin Imaging. 1998. 22:15–22.
9. Putnam JB Jr, Sweeney MS, Colon R, Lanza LA, Frazier OH, Cooley DA. Primary cardiac sarcomas. Ann Thorac Surg. 1991. 51:906–910.
10. Min DJ, Kang DH, Seung KB, et al. A primary cardiac angiosarcoma. Korean Circ J. 1995. 25:704–709.
11. Kim JS, Song SG, Ko WS, et al. A case of primary right atrial angiosarcoma manifested with cardiac tamponade. J Korean Soc Echocardiogr. 2004. 12:36–38.
12. Llombart-Cussac A, Pivot X, Contesso G, et al. Adjuvant chemotherapy for primary cardiac sarcomas: the IGR experience. Br J Cancer. 1998. 78:1624–1628.
13. Alvarez JM, Yew MK, Rajesh B, Ireland M. Cardiac angiosarcoma: too little known, too late treatment or just too bad a tumour? Heart Lung Circ. 2001. 10:30–34.
14. Donsbeck AV, Ranchere D, Coindre JM, Le Gall F, Cordier JF, Loire R. Primary cardiac sarcomas: an immunohistochemical and grading study with long-term follow-up of 24 cases. Histopathology. 1999. 34:295–304.
15. Talbot SM, Taub RN, Keohan ML, Edwards N, Galantowicz ME, Schulman LL. Combined heart and lung transplantation for unresectable primary cardiac sarcoma. J Thorac Cardiovasc Surg. 2002. 124:1145–1148.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download