Abstract
Background and Objectives
Coronary artery disease (CAD) is a major cause of heart failure associated with left ventricular systolic dysfunction (LVSD). The prognosis of LVSD is significantly influenced by the etiology of heart failure and therefore, differentiation of significant CAD from other etiologies is important. Carotid intima-media thickness (IMT) and plaque are useful predictors for cardiovascular events, including stroke and CAD. The purpose of this study was to evaluate the predictive value of carotid IMT and plaque for the diagnosis of CAD in LVSD patients.
Subjects and Methods
Seventy-three (n= 73, 47 male, 67.6±12.4 years) patients hospitalized for heart failure with severe LVSD were retrospectively enrolled. The severity of CAD was analyzed by the Duke Jeopardy Score system, and carotid IMT and plaque were measured according to the Mannheim Carotid IMT Consensus.
Results
Significant CAD was found in 41 patients (56.1%, CAD group) on coronary angiography. Mean common carotid artery (CCA) IMT (0.74±0.05 mm vs. 1.04±0.04 mm, p<0.01) was significantly higher in the CAD group. Plaque in CCA (6.25% vs. 19.5%, p<0.01) and plaque in bulb (25.0% vs. 60.9%, p<0.001) were significantly higher in the CAD group. Mean CCA IMT {odds ratio (OR) 2.61, 95% confidence interval (CI) 1.134-4.469, p<0.01} and plaque in bulb (OR 4.69, 95% CI 1.702-12.965, p<0.01) were significant predictors for the diagnosis of CAD according to multivariate logistic regression analysis.
Coronary artery disease (CAD) is one of the most important causes of left ventricular systolic dysfunction (LVSD) and it is found in approximately 68% of patients with LVSD.1) Because left ventricular (LV) function and prognosis can be improved with successful revascularization, differentiation of significant CAD from other non-ischemic etiologies is important in managing these patients. However, in advanced heart failure, it is sometimes difficult to delineate the underlying etiology with echocardiography, because of the features of dilated cardiomyopathy. Patients with dilated cardiomyopathy may present with chest pain or ECG change suggestive of CAD, whereas some patients with CAD and heart failure present without history of angina or electrocardiography (ECG) evidence of myocardial ischemia/infaction (Fig. 1). Conventional coronary angiography is the gold standard method for the diagnosis of CAD. However, it is invasive and associated with nephrotoxicity and other risks. Some patients strongly refuse to undergo invasive coronary angiography. This has led to the development of non-invasive imaging for the diagnosis of CAD, which often involves expensive equipment, radiation exposure, needs medication and contrast administration.2) Carotid intima-media thickness (IMT) is efficient, relatively inexpensive, highly reproducible and does not expose patients to contrast dye and radiation. In addition, carotid IMT is a well established surrogate marker of atherosclerosis and is associated with cardiovascular events and asymptomatic myocardial ischemia.3) Recently, some reports discussed the differentiation of ischemic cardiomyopathy from dilated cardiomyopathy using carotid ultrasonography.4)5) As such, the aim of this study was to explore the predictive value of carotid IMT and plaque for the diagnosis of CAD in severe LVSD patients.
Between August 2005 and May 2009, 181 newly diagnosed heart failure patients were admitted with LV dilatation {left ventricular end-diastolic dimension (LVEDd) >55 mm} and severe LVSD {left ventricular ejection fraction (LVEF) ≤30% with modified Simpson's method}. We excluded 70 patients with previous history of myocardial infaction (MI), percutaneous coronary intervention (PCI), coronary artery bypass graft (CABG), acute coronary syndrome (ACS) and elevated cardiac enzyme on admission. In addition, we also excluded 38 patients who did not undergo diagnostic work up for coronary anatomy. Seventy-three (n=73) patients underwent coronary arteriography, conventional coronary angiography (64 patients) and CT coronary angiography (9 patients) were included in this study. Decision to perform diagnostic work up for coronary anatomy was at the duty physicians' discretion, according to the patients' clinical features at presentation, including visible Q wave or poor R wave progression on ECG, presence of cardiovascular risk factors and associated chest pain. We classified patients into two groups: the CAD group and the non-CAD group, according to coronary angiographic findings.
Two dimensional images were obtained using standard views in the left lateral decubitus position. LV dimensions were obtained in the standard views. LV end systole and end diastolic volumes were calculated by using the modified Simpson's method, and ejection fraction was calculated from the LV end systole and end diastolic volumes.
Carotid ultrasound examinations were conducted using commercially available linear array transducer (8.0 MHz linear probe, Acuson sequoia C 512, Siemens, automated measurement). Experienced sonographer obtained images of the far wall of both common carotid artery (CCA) and carotid bulbs according to the Mannheim common carotid IMT consensus. Plaque was defined as a focal structure encroaching into the arterial lumen of at least 0.5 mm or 50% of the surrounding IMT value, or demonstrated a thickness greater than 1.5 mm as measured from the media-adventitia interface to the intima-lumen interface.6)
Each CCA segment was measured and these measurements on both sides were averaged to obtain mean CCA IMT. Thickness greater than 0.9 mm was regarded as increased CCA IMT. We also considered the presence of plaque on either side as positive finding.
Coronary angiography was performed via the femoral or radial artery using standard technique, and CT coronary angiography was obtained using 64 channel multi detector CT scanner (Lightspeed VCT XT, GE Healthcare, USA). CAD was defined as greater than 70% stenosis in a major epicardial coronary artery. We used the Duke Myocardial Jeopardy Score to evaluate the severity of CAD, categorized as mild (jeopardy score 2-4 points), moderate (6-8 points), severe (10-12 points). In this jeopardy scoring system, the coronary tree is divided into 6 segments: the left anterior descending artery (LAD), diagonal branches, septal perforating branches, circumflex coronary artery, obtuse marginal branches, and the posterior descending coronary artery. Segments distal to stenoses greater than 70% are considered to be 'at risk'. Each such segment is assigned 2 points. The maximum possible number of points is 12.7) For example, significant proximal LAD stenosis makes 6 jeopardy score points (septal perforator: 2 points, diagonal branch: 2 points and 2 points for LAD). Single vessel disease, for example due to right coronary artery (RCA) stenosis, will be awarded 2 jeopardy score points.
Data are expressed as mean±SD and frequencies are expressed as percentages. The differences between measurements were tested using t-test for continuous variables and Chi-square test for categorical variables. A probability value of p<0.05 was considered statistically significant. Multivariate logistic regression analysis was performed to determine the independent predictor for CAD. Data were analyzed by using Statview for Windows, version 5.0 (SAS Institute, Inc., Cary, NC, USA), and MedCalc, version 7.0 (MedCalc Software, Mariakerke, Belgium).
Among the study cohort of 73 patients, there was no significant stenosis in 32 patients (jeopardy score= 0, Non-CAD group), whereas 41 patients experienced significant stenosis (jeopardy score ≥2, CAD group). Three patients were awarded 2 points on the jeopardy score, because all of whom experienced significant stenosis in the RCA. Baseline characteristics of the two groups are summarized in Table 1. There were no significant differences in age (68.3±13.7 years vs. 70.4±13.1 years), male sex (40.4% vs. 59.6%), diabetes (25% vs. 46.3%), smoking (31% vs. 48.3%), LVEDd (63.4±6.2 mm vs. 63.1±7.5 mm), LVEF (22.6±4.6% vs. 24.0±4.7%). The prevalence of hypertension (34.3% vs. 65.8%, p<0.01) and Q wave in V1-4 (6.3% vs. 14.3%, p<0.01) was significantly higher in the CAD group.
Mean CCA IMT was significantly higher in the CAD group than in the non-CAD group (0.74±0.05 mm vs. 1.04±0.04 mm, p<0.01) (Figs. 1 and 2A). Comparing patients with jeopardy score between 2 and 4 points (the mild group), and patients with jeopardy score between 10 and 12 points (the severe group), the latter demonstrated more increased CCA IMT (0.86±0.15 mm vs. 1.04±0.24 mm, p<0.05) (Fig. 2B). Plaque in CCA (6.25% vs. 19.5%, p<0.01), and plaque in bulb (25.0% vs. 60.9%, p<0.001) were more frequently found in the CAD group (Table 2).
Receiver-operating characteristic curves constructed for different values of mean CCA IMT versus CAD demonstrated the optimal mean CCA IMT cut off value for ischemic etiology (Fig. 3). The area under curve was 0.741 and a cut off value of CCA IMT at 0.9 mm had sensitivity and specificity of respectively 56.1% and 88.2% in detecting CAD and that of a cut off value of 1.0 mm was respectively 35.9% and 94.1% (Fig. 3). The sensitivity and specificity of plaque in CCA in detecting CAD were respectively 19.5% and 93.7% and that for plaque in bulb was respectively 61% and 75% (Table 3).
Multivariate logistic regression analysis showed that hypertension {odds ratio (OR) 3.0, 95% confidence interval (CI) 1.12-11.29, p<0.05}, mean CCA IMT (OR 2.61, 95% CI 1.134-4.469, p<0.01) and plaque in bulb (OR 4.69, 95% CI 1.702-12.965, p<0.01) were significant predictors for CAD (Table 4).
Among 29 patients classified with low probability for CAD (no history of diabetes mellitus, no Q wave in ECG), 8 patients (27.6%) were shown to have significant CAD (jeopardy score 4 in 3, 8 in 2 and 10 in 3 patients). The presence of plaque in bulb was a significant predictor for CAD (OR 7.08, 95% CI 1.17-42.8, p<0.05) in patients classified as low probability (Table 5).
In daily medical practice, it remains a challenge to distinguish between ischemic and non-ischemic cardiomyopathy. Ischemic etiology has been shown to be independently associated with worse long-term outcome in patients with LVSD.8) The etiology of cardiomyopathy also influences the decision to pursue revascularization and the choice of pharmacologic intervention.9)
Dilated cardiomyopathy, defined as LVSD with chamber dilatation, represents a final common pathway for many pathologic processes. No clear etiology can be identified in a substantial proportion of cases. For example, 8% of CAD was found in transplantation candidates diagnosed with idiopathic dilated cardiomyopathy.10) In patients with definite history of MI, PCI, CABG, ACS and elevated cardiac marker, there was no uncertainty on the ischemic etiology of cardiomyopathy. We did not hesitate to perform coronary angiography, unless patients refuse invasive procedure. However, in certain patients, determination of etiology may be difficult because patients with heart failure without CAD may present with typical angina or regional wall motion abnormalities on echocardiography, whereas patients with severe CAD may present without symptom of angina or history of myocardial ischemia/infaction. Carotid IMT is a well-established surrogate marker of coronary atherosclerosis,11)12) and is associated with cardiovascular events.13) It is efficient, relatively inexpensive and highly reproducible and does not expose patients to contrast dye or radiation. Previous studies demonstrated the relationship between carotid IMT and the extent and severity of coronary stenosis.14-19) Therefore, it can be postulated that carotid IMT and plaque provide diagnostic clue for ischemic etiology in severe LVSD patients. Atherosclerosis is a systemic disease and, as such, increasing carotid IMT and plaque are correlated with CAD. However, this association remains debatable.20) Our study demonstrated that mean CCA IMT is higher in the CAD group (Fig. 2) and mean CCA IMT is increasing according to coronary jeopardy score, which is a simple method for estimating the amount of myocardium at risk on the basis of coronary artery stenosis. Even after having excluded patients with known ischemic history, significant CAD was found to exist in 41 patients (56.1%) in our study population. Among 29 patients classified with low probability of developing CAD, CAD was found to exist in 8 patients (27.6%) and plaque in bulb remained statistically significant predictor for CAD. We therefore conclude that detection of CAD of unknown etiology in severe LVSD patients is important, because they need revascularization that can improve prognosis. In addition, our study demonstrated showed good specificity of mean CCA IMT (93.7%) (Fig. 3) and the presence of plaque in bulb also exhibited relatively good specificity (75%) and positive predictive value (80%) for the diagnosis of CAD (Table 3). Therefore, we weighted on mean CCA IMT or the presence of plaque can be used as tools to support the presence of ischemic etiology in severe LVSD patients and normal CCA IMT in the absence of plaque support the non-ischemic etiology of severe LVSD patients. In practice, if severe LVSD patients have no history of ischemia with normal IMT and without plaque in bulb, we can initially classify them as non-ischemic LVSD. On the contrary, increased IMT with plaque in bulb in patients are indications to perform coronary angiography.
Based on our findings, mean CCA IMT and plaque in bulb can be useful tools for the prediction of CAD in severe LVSD patients with unknown etiology.
This study is limited by the small sample size and its retrospective nature. In addition, there might have been inherent selection bias because we did not include all patients with severe LVSD. It may not be clinically justified to perform coronary angiography in all patients due to the low probability of atherosclerosis, particularly in young patients.
Mean CCA IMT was higher with CAD group in severe LVSD patients. And mean CCA IMT and plaque in bulb were good predictors for CAD. CCA IMT and plaque in bulb showed good specificity and positive predictive value for CAD. In patients with severe LVSD, mean CCA IMT and plaque in bulb can be useful additional tools for the prediction and/or exclusion for CAD.
Figures and Tables
Fig. 1
Representative image of carotid intima-media thickness, and coronary angiography in severe left ventricular systolic dysfunction patients. A and B: non-CAD patient. C and D: CAD patient. CAD: coronary artery disease.
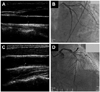
Fig. 2
Mean common carotid artery intima-media thickness (CCA-IMT) in CAD and Non-CAD group (A) and mild, moderate, severe CAD patients (B). CAD: coronary artery disease.

Fig. 3
Receiver operating characteristic curve for mean CCA IMT versus CAD. CCA: common carotid artery, IMT: intima-media thickness, CAD: coronary artery disease.

Acknowledgments
This study was supported by the 2008 Konkuk University Medical Center Research Grant.
References
1. Gheorghiade M, Bonow RO. Chronic heart failure in the United States: a manifestation of coronary artery disease. Circulation. 1998. 97:282–289.
2. Feinstein SB, Voci P, Pizzuto F. Noninvasive surrogate markers of atherosclerosis. Am J Cardiol. 2002. 89:31C–43C.
3. Nagai Y, Metter EJ, Earley CJ, et al. Increased carotid artery intimal-medial thickness in asymptomatic older subjects with exercise-induced myocardial ischemia. Circulation. 1998. 98:1504–1509.
4. Reynolds HR, Steckman DA, Tunick PA, Kronzon I, Lobach I, Rosenzweig BP. Normal intima-media thickness on carotid ultrasound reliably excludes an ischemic cause of cardiomyopathy. Am Heart J. 2010. 159:1059–1066.
5. Tamura T, Nojiri A, Sakamoto H, Kurusu O, Mochizuki S. Ultrasonographic assessment of carotid atherosclerosis for the differentiation of ischemic cardiomyopathy and dilated cardiomyopathy. J Cardiol. 2005. 46:97–103.
6. Touboul PJ, Hennerici MG, Meairs S, et al. Mannheim carotid intima-media thickness consensus (2004-2006). An update on behalf of the Advisory Board of the 3rd and 4th Watching the Risk Symposium, 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovasc Dis. 2007. 23:75–80.
7. Califf RM, Phillips HR 3rd, Hindman MC, et al. Prognostic value of a coronary artery jeopardy score. J Am Coll Cardiol. 1985. 5:1055–1063.
8. Felker GM, Shaw LK, O'Connor CM. A standardized definition of ischemic cardiomyopathy for use in clinical research. J Am Coll Cardiol. 2002. 39:210–218.
9. Follath F, Cleland JG, Klein W, Murphy R. Etiology and response to drug treatment in heart failure. J Am Coll Cardiol. 1998. 32:1167–1172.
10. Felker GM, Hu W, Hare JM, Hruban RH, Baughman KL, Kasper EK. The spectrum of dilated cardiomyopathy. The Johns Hopkins experience with 1,278 patients. Medicine Baltimore. 1999. 78:270–283.
11. Kwon TG, Kim KW, Park HW, Jeong JH, Kim KY, Bae JH. Prevalence and significance of carotid plaques in patients with coronary atherosclerosis. Korean Circ J. 2009. 39:317–321.
12. Sinha AK, Eigenbrodt M, Mehta JL. Does carotid intima media thickness indicate coronary atherosclerosis? Curr Opin Cardiol. 2002. 17:526–530.
13. Hodis HN, Mack WJ, LaBree L, et al. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med. 1998. 128:262–269.
14. Hallerstam S, Larsson PT, Zuber E, Rosfors S. Carotid atherosclerosis is correlated with extent and severity of coronary artery disease evaluated by myocardial perfusion scintigraphy. Angiology. 2004. 55:281–288.
15. Lekakis JP, Papamichael CM, Cimponeriu AT, et al. Atherosclerotic changes of extracoronary arteries are associated with the extent of coronary atherosclerosis. Am J Cardiol. 2000. 85:949–952.
16. Kim JH, Yoon HJ, Hong EJ, et al. Clinical significance of B-mode ultrasound of common carotid artery for prediction of severity of coronary artery disease: important parameters on hand measurement. Korean Circ J. 2005. 35:467–473.
17. Kablak-Ziembicka A, Tracz W, Przewlocki T, Pieniazek P, Sokolowski A, Konieczynska M. Association of increased carotid intima-media thickness with the extent of coronary artery disease. Heart. 2004. 90:1286–1290.
18. Wofford JL, Kahl FR, Howard GR, McKinney WM, Toole JF, Crouse JR 3rd. Relation of extent of extracranial carotid artery atherosclerosis as measured by B-mode ultrasound to the extent of coronary atherosclerosis. Arterioscler Thromb. 1991. 11:1786–1794.
19. Lekakis JP, Papamichael C, Papaioannou TG, et al. Intima-media thickness score from carotid and femoral arteries predicts the extent of coronary artery disease: intima-media thickness and CAD. Int J Cardiovasc Imaging. 2005. 21:495–501.
20. Bots ML, Baldassarre D, Simon A, et al. Carotid intima-media thickness and coronary atherosclerosis: weak or strong relations? Eur Heart. 2007. 28:398–406.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download