Abstract
Background and Objectives
The thioredoxin (TRx) system is a ubiquitous thiol oxidoreductase pathway that regulates cellular reduction/oxidation status. Although endothelial cell (EC) hypoxic damage is one of the important pathophysiologic mechanisms of ischemic heart disease, its relationship to the temporal expression pattern of the TRx system has not yet been elucidated well. The work presented here was performed to define the expression pattern of the TRx system and its correlation with cellular apoptosis in EC lines in hypoxic stress. These results should provide basic clues for applying aspects of the TRx system as a therapeutic molecule in cardiovascular diseases.
Subjects and Methods
Hypoxia was induced with 1% O2, generated in a BBL GasPak Pouch (Becton Dickinson, Franklin Lakes, NJ, USA) in human endothelial progenitor cells (hEPC) and human umbilical vein endothelial cells (HUVEC). Apoptosis of these cells was confirmed by Annexin-V: Phycoerythrin flow cytometry. Expression patterns of TRx; TRx reductase; TRx interacting protein; and survival signals, such as Bcl-2 and Bax, in ECs under hypoxia were checked.
Results
Apoptosis was evident after hypoxia in the two cell types. Higher TRx expression was observed at 12 hours after hypoxia in hEPCs and 12, 36, 72 hours of hypoxia in HUVECs. The expression patterns of the TRx system components showed correlation with EC apoptosis and cell survival markers.
Conclusion
Hypoxia induced significant apoptosis and its related active changes of the TRx system were evident in human EC lines. If the cellular impact of TRx expression pattern in various cardiovascular tissues under hypoxia or oxidative stress was studied meticulously, the TRx system could be applied as a new therapeutic target in cardiovascular diseases, such as ischemic heart disease or atherosclerosis.
Oxidative stress is associated with various pathophysological states, such as chronic inflammatory diseases, malignancies, and neurodegenerative disorders.1-3) A number of studies have recently reported that oxidative stress also has important roles in the development and advance of hypertension, atherosclerosis, myocardial ischemia, and restenosis after coronary intervention.3-5) There are various kinds of reactive oxygen species (ROS) that are correlated with some cardiovascular diseases, such as nitric oxide (NO·), superoxide (O2·-), hydrogen peroxide (H2O2), and peroxinitrite (ONOO·-).3) NO· is normally produced by endothelial nitric oxide synthase (NOS) in the vascular endothelium, and inducible NOS is expressed in macrophages and smooth muscle cells. Superoxide is generated by a one electron reduction of oxygen by various forms of oxidases such as xanthine oxidase, Nicotinamide Adenine Dinucleotide Phosphate (NADPH) oxidase, mitochondrial cytochrome P450 monooxygenase, heme oxygenase, and lipoxygenase.3) Peroxinitrite is rapidly generated by the reaction of NO· with O2·-.3)
Because ROS have various harmful effects on subcellular organelles, a counterpart system to limit ROS action, known as the antioxidative system, is essential for cellular survival.
The thioredoxin (TRx) system is one of the crucial antioxidative pathways and is a thiol-reducing system which is composed of TRx, TRx reductase (TRxR), and TRx interacting protein (TxNip). It reduces oxidized cystein groups on proteins through an interaction with the redox-active center of TRx.6) TRx reduces the oxidized form of TRx peroxidase, and the reduced TRx eliminates ROS.
Endothelial cells (ECs) of the arterial intima constitute the crucial contact surface with blood. ECs and possess many highly regulated mechanisms to maintain vascular homeostasis that often go awry during the pathogenesis of vascular diseases. For example, ECs show antithrombotic action via expression of prostacyclin, heparan sulfate proteoglycan, and plasminogen activator inhibitor-1.9) ECs also secrete NO to control vascular tone and contractility. During EC desquamation, mobilized human endothelial progenitor cells (hEPC) from bone marrow populate the injured intima area.9) This function is closely related with the, severity of atherosclerosis, diabetes mellitus, smoking and age.10) TRx is expressed ubiquitously in ECs and protects ECs from various kinds of oxidative stresses.11) H2O2 increases TRx expression in ECs and low concentrations of H2O2 protect ECs from apoptosis by induction of TRx overexpression; however, high concentrations of H2O2 will exhaust the TRx reservoir and eventually induce EC apoptosis.12) Up to now, there has been little data available on the temporal expression pattern of the TRx system and its correlation with apoptosis in ECs under hypoxia. In this report, we describe the hypoxia-induced temporal expression of the TRx system, its correlation with apoptosis, and survival signal changes in hEPCs and human umbilical vein endothelial cells (HUVECs). We expected that this work will provide us the optimal time condition of TRx therapy such as gene delivery of TRx to overcome various cardiovascular diseases related with EC pathology in animal models.
hEPCs were isolated from human peripheral blood with minor modification of the protocol of Kalka et al.13) Briefly, the mononuclear cells were fractionated from other components of peripheral blood by centrifugation at 1,200 rpm for 10 minutes on Histopaque-1077 (Sigma-Aldrich Co., St. Louis, MO, USA) gradients according to manufacturer's instructions. Isolated mononuclear cells were resuspended using an EGM-2 BulletKit system (Lonza Ltd., Basel, Switzerland) consisting of endothelial basal medium, 5% fetal bovine serum (FBS), human endothelial growth factor, vascular endothelial growth factor, human fibroblast growth factor-B, insulin-like growth factor-1, ascorbic acid and heparin. Mononuclear cells were seeded at 1×107 cells per well on 2% gelatin-coated six-well plates (Sigma-Aldrich Co.). The media was changed on the 6th day after plating and every 3 days thereafter. Each cluster or colony was observed microscopically daily. Cells were passaged at confluence following dissociation with 0.05% trypsin and grown for 3 days in fresh medium which was consisted of M199 (Sigma-Aldrich Co.) supplemented with 10% FBS and antibiotics or the EGM-2 BulletKit system.
HUVECs were isolated from human umbilical veins, as previously described.14) Umbilical cords were immersed into a culture dish and carried to the cell culture room. Both ends of the cord were bound and the ends of the umbilical vein were cannulated. The vein was washed through the cannula by sterile phosphate buffered saline (PBS). Then, the vein was filled with 0.02% collagenase in PBS, and placed in a CO2 incubator for 10 minutes at 37℃. HUVECs were isolated in centrifuge tube from the vessel by two times of washing with 10 mL M199. Cell suspension was centrifuged at 1,200 rpm for 10 minutes, and the supernatant was discarded and the cells were resuspended with EGM-2 BulletKit system on 2% gelatin-coated six-well culturing plates. Cells were passaged at confluence following dissociation with 0.05% trypsin and grown for 3 days in fresh medium which was consisted of M199 supplemented with 10% FBS and antibiotics or EGM-2 BulletKit system.
To induce the hypoxic condition in the two EC lines, cultured hEPC and HUVEC were placed in the GasPak™ pouch system (BD, Franklin Lakes, NJ, USA) according to the manufacturer's instructions. The GasPak pouch system was maintained in a CO2 incubator 37℃ until cell harvesting at 6, 12, 24, 36, 48, 60 and 72 hours.
Cultured hEPC and HUVEC were processed with the Annexin V: Phycoerythrin (PE) Apoptosis Detection Kit I (BD). The Annexin V: PE Kit was used to assess cell membrane integrity; 7-Aminoacetinomycin D (AAD, BD) was used to assess cell membrane permeability, and thus cell apoptosis. In brief, collected hEPC and HUVEC were washed twice with cold PBS and then resuspended in the binding buffer (10 mM HEPES, 140 mM NaCl, 2.5 mM CaCl2, pH 7.4) at a concentration 5×106 cell/mL. After these mixtures were transferred to a 5 mL culture tube, 5 µL of Annexin-V and 7-AAD were added. After gentle vortexing, cells were incubated for 10 minutes at room temperature in the dark and analyzed by fluorescence-activated cell sorting (FACS). The FACSCalibur-S (BD) was equipped with an argon ion laser system which was adjusted to 488 nm excitation and 578 nm maximum emission. FACS analysis was performed 4 times on each cell line and time point. Results were depicted as mean±standard deviation (SD). The proportion of apoptosis in the control (baseline time) was described as 100% in each cell line, the percent change in degree of apoptosis at each observation time was calculated and depicted by comparing it to apoptosis in the control.
To examine the expression pattern of the TRx system in hEPCs and HUVECs which were stressed by hypoxia, we performed a Western blot analysis. Harvested hEPCs and HUVECs were immersed in PRO-PREP Protein Extraction Solution (iNtRON Biotechnology, Seongnam, Korea) and incubated at 4℃ for 2 hours. The extracts were centrifuged with 12,000 rpm at 4℃ for 15 minutes to remove insoluble material. Protein concentrations were determined using the Bio-Rad protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA). Equal amounts of total protein (20 µg) were diluted with sample buffer containing 100 mM dithiothreitol and heated to 98℃ for 5 minutes and subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis in a gel apparatus (Hoefer, Holliston, MA, USA), subsequently transferred to polyvinylidene fluoride membranes according to the method of Gershoni and Palade15) Blots were blocked with 5% skim milk in Tris-Buffered Saline Tween-20 (TBST) for 2 hours (at RT or 4℃). The membrane was incubated with antibodies against TRx, TRxR, TxNip, Bcl-2, Bax-α, Akt, Survivin (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The antibodies were diluted 1 : 2,000 in TBST, incubated, shaking, at room temperature for 1 hour, washed in TBST, and incubated with horseradish peroxidase-conjugated secondary antibody (1 : 2,000-1 : 5,000) for 1 hour. After washing, blots were developed using the West-Zol kit (iNtRON Biotechnology) for 1 minute and chemiluminescence was detected using the LAS-3000 (Fujifilm, Minato, Tokyo, Japan) for 10-300 seconds. Optical density (OD) was evaluated by the MultiGauge Ver 3.1 (Fujifilm) program. ODs were calculated 5 times for each result and expressed as mean±SD. OD in the control (baseline time) was described as 100% in each cell line, and the percent change in OD at each observation time was calculated and depicted by comparing it to the OD of the control.
Total ribonucleic acid (RNA) was extracted from cultured cells using TRIzol® Reagent (Invitrogen, San Diego, CA, USA) according to the manufacturer's instructions. Gene-specific primers for the human TRx gene (forward, 5'-TTCTTTCATTCCCTCTGTG-3'; reverse, 5'-TCCGTAATAGTGGCTTCG-3') and human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (forward, 5'-ATGGGGAAGGTGAAGGTCGGAGTC-3'; reverse, 5'-CCATGCCAGTGAGCTTCCCGTTC-3') were synthesized (BIONEER, Daejeon, Korea). RNA template and primers mixed Maxime™ reverse transcription-polymerase chain reaction PreMix (iNtRON). PCR products were separated on a 1.5% agarose gel and imaged on an SL-20 deoxyribonucleic acid Image Visualizer (SeouLin Bioscience, Seoul, Korea). ODs were evaluated using the MultiGauge Ver 3.1 (Fujifilm) program. Each OD was calculated 3 times and expressed as mean±SD. The OD of the control (baseline time) was describe as 100% in each cell line, percent change in OD at each observation time was calculated and depicted by comparing each to the OD of the control.
The pattern of cellular apoptosis was examined with the Annexin V: PE FACS in hEPC and HUVEC along with the duration of hypoxia. hEPC showed the typical bimodal peaks of the apoptosis pattern during hypoxia. At 12 and 24 hours after the initiation of hypoxia, the proportion of apoptotic hEPCs increased steeply to 175.9±35.8%, and 398.3±75.8%, respectively, compared with nonhypoxic control cells (100±23.7%, p<0.05 at 12 and 24 hours) (Fig. 1). The level of apoptosis at 36 and 48 hours of hypoxia was significantly decreased when compared with that at 24 hours of hypoxia; however, the level of apoptosis had increased at 60 and 72 hours of hypoxia when compared with control (220.5±68.1%, 368.8±20.3%, p<0.05) (Fig. 1).
The apoptosis pattern of HUVECs under hypoxic stress was slightly different from that of hEPCs. The initial peak of apoptosis occurred at 12 hours of hypoxia, (256.3±42.2%) compared with control cells (100±25.2%, p<0.05). Apoptosis was decreased to nadir at 36 hours of hypoxia, after which the level of apoptosis increased again up to 315.2±42.3% at 48 hours, 202.3±36.9% at 60 hours, and 500.6±45.6% at 72 hours of hypoxia (Fig. 1).
TRx expression in hEPCs was significantly increased compared with control at 12 hours (651.3±78.5% vs. 100.0±24.5%, p<0.05) to 72 hours of hypoxia (898.7±145.2% vs. 100±24.5%, p<0.05) (Fig. 2). TRxR expression remained low up to 36 hours under hypoxia, but had increased abruptly at 48 hours when compared with control (1,320.1±127.9% vs. 100±24.2%, p<0.05) (Fig. 2).
In HUVECs under hypoxic stress, three significant peaks of TRx expression were noticed at 12, 36, and 72 hours of hypoxia (640.1±89.4%, 620.2±98.1%, 595.9±128.4% vs. 100±19.7%, p<0.05 compared to control at each time point) (Fig. 2). TxNip in HUVECs under hypoxia showed apattern of expression similar to that of TRx from 12 to 48 hours of hypoxia, but nadir expression was continued thereafter. TRxR expression in HUVECs decreased steadily during the period of hypoxic stress.
The TRx/TxNip ratio, a marker of TRx system activation in hypoxia, showed different patterns in the 2 EC lines. In hEPCs, the TRx/TxNip ratio was significantly increased at 36 hours of hypoxia compared with control (250.8±77.8% vs. 100±35.4%, p<0.05) (Fig. 2). In HUVECs, the TRx/TxNip ratio steadily increased from 6 hours onward, and was significantly increased at 48 hours and 72 hours when compared with control (301.7±49.2%, 550.4±78.4% vs. 100±24.3%, p<0.05 on 48, 72 hours) (Fig. 2).
The temporal expression patterns of these 2 EC lines under hypoxic stress were very distinct. hEPCs showed significant increases in TRx messenger ribonucleic acid (mRNA) expression at 24, 36, and 48 hours of hypoxia compared with control (193.5±56.3%, 188.7±65.9, 205.9±61.2% vs. 100±20.0%, p<0.05 at each time point) (Fig. 3). No distinct changes in expression of TRx mRNA were observed in HUVECs (Fig. 3).
In hEPCs under hypoxic stress, Bcl-2 expression was markedly increased at 12 and 60 hours of hypoxia (182.7±48.3%, 189.5±52.9% vs. 100±24.2%, p<0.05 at each time point) (Fig. 4). Lower values of Bcl-2 expression were observed from 24 to 48 hours and 72 hours of hypoxia, which was well correlated with the degree of apoptosis seen in hEPCs.
In HUVECs, Bcl-2 expression was higher at 6, 12, 36, 60, 72 hours of hypoxia (259.4±44.9%, 243.4±38.7%, 274.8±46.8%, 220.9±49.7%, 279.3±50.1% vs. 100±7.96%, p<0.05 at each time point) and the expression of Bax was similar.
The Bax/Bcl-2 ratio, as a marker of apoptosis/survival, showed a similar pattern during hypoxia stress in the two cell lines. However, significantly higher values were observed in hEPCs at 24 (212.4±67.0% vs. 110.8±48.3%, p<0.05) and 60 hours (275.9±86.3% vs. 89.5±59.1%, p<0.05) of hypoxia when compared with HUVECs (Fig. 4).
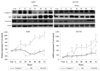
Only slight increases in the expression of caspase 3 in hEPCs were observed at 12 and 60 hours of hypoxia. Survivin expression was increased at 12 hours of hypoxia. Akt expression increased during the whole period of hypoxia in hEPCs.
Slight and steady increases of caspase 3 expression in HUVECs were observed, with expression at 48, 60, 72 hours of hypoxia significantly elevated compared with control at these time points (196.8±45.9%, 276.7±78.2%, 289.6±89.4% vs. 100±24.1%, p<0.05) (Fig. 5). Akt was significantly over-expressed from 24 hours of hypoxia and thereafter; and elevated expression of survivin was observed at 12, 60, and 72 hours of hypoxia in HUVECs (Fig. 5). Grossly, the expression patterns of these 3 proteins were very similar between two ECs lines (Fig. 5).
ECs have putative roles in maintaining the vascular homeostasis. ECs lining the vascular endothelium release various vasodilators, including NO and prostacyclin, as well as several kinds of vasoconstrictors.15) ECs also control the intravascular coagulation-fibrinolysis system to maintain a non-thrombotic milieu.
Endothelial dysfunction is an early pathologic feature in cardiovascular diseases. An imbalance between oxidative stress and the anti-oxidative system in ECs is a common underlying cause of cardiovascular disease. Several lines of evidence have accumulated that support an important role for oxidative stress in the pathogenesis and aggravation of various cardiovascular diseases, such as hypertension, dyslipidemia, diabetes mellitus, atherosclerosis, ventricular hypertrophy, ischemic heart disease, and heart failure.16-20) The susceptibility of cell lines of cardiovascular tissues such as EC, cardiomyocytes, and fibroblasts to oxidative stress depends on the balance between the generation of ROS, described earlier, and the scavenging activity of the antioxidative system. One important antioxidative mechanism in mammalian cells is the TRx system which is a thiol reducing system that acts against ROS, especially NO and H2O2.
TRx is a small, ubiquitous thiol protein and is one of the important regulators of the reduction-oxidation balance and redox-controlled cell functions. Regulation of TRx is closely correlated with the control of cellular redox balance, the promotion of cell growth, the inhibition of apoptosis, and the modulation of inflammation. The redox activity of TRx totally depends on two cystein residues of Cys32 and Cys35.21) The two cystein residues exist as a dithiol in the reduced form and a disulfide in the oxidized form. TRx is oxidized when it transfers reducing equivalents to disulfide groups in target proteins and it is reduced back to the dithiol form by an NADPH-dependent protein, TRxR.22) Moreover, TRx is expressed prominently in ECs and protects ECs from H2O2-induced cytotoxicity.23) ROS induce TRx expression in ECs and low concentrations of H2O2 protect ECs from apoptosis via overexpression of TRx.24)
Another important molecule in the TRx system is TxNip. It inhibits TRx activity by interacting with the catalytic site of TRx, suggesting that TxNip is an endogenous inhibitor of TRx.25) Hyperglycemia induces overexpression of TxNip in vascular tissue and reduces TRx activity,26) implicating a role for oxidative stress in diabetes TxNip also has important role in cardiovascular disorders and acts as a sensor for biomechanical and oxidative stress.27) Therefore, the antioxidative capability of the TRx system mainly depends on the action and expression pattern of TRx, TRxR, and TxNip. In hEPCs, TRx expression rapidly increased at 12 hours of hypoxia and this time point was closely related with increased apoptosis in these cells. However, the increase in TRx mRNA expression was also observed at 24 hours of hypoxia, making the interval from 12 to 24 hours of hypoxia was exhausted period of TRx and higher apoptosis on 24 hours of hEPC could be analogized.
TxNip is an endogenous inhibitor of TRx, so the TRx/TxNip ratio can be used as an gross indicator for the antioxidative capacity of the TRx system. The TRx/TxNip ratio in hEPCs showed its lowest level up to 36 hours of hypoxia and this period could be considered as the utilization phase of the cytosolic form of TRx. The mRNA level of TRx was elevated at 24 hours of hypoxia and marked TRx elevation was observed at 36 hours of hypoxia at a level which could prevent hEPC apoptosis.
In HUVECs, there were three peaks of TRx expression at 12, 36, and 72 hours of hypoxia. These patterns of TRx expression were relatively well correlated with increases in apoptosis in HUVECs. The expression of TRxR was reduced compared with control and the expression of TxNip was also not evident during whole period of hypoxia in HUVECs. The expression of TRx mRNA and TRx protein during hypoxia in HUVECs showed marked discrepancy and the expression pattern of TRx in the two cell lines was also very distinct.
The TRx/TxNip ratio in HUVECs showed two peaks at 48 and 72 hours of hypoxia, a pattern that was relatively well matched with the apoptosis pattern in HUVECs. Contrary to hEPCs, the TRx and TRx/TxNip ratio was well correlated with the degree of apoptosis in HUVECs, but not with apoptosis prevention. The higher Bax/Bcl-2 ratio in HUVECs was observed at 24 and 60 hours of hypoxia, and a lower Bax/Bcl-2 ratio was observed at 6 and 48 hours of hypoxia; these finding were relatively well correlated with the degree of apoptosis in HUVECs during hypoxia. According to these findings, the TRx system expression pattern shown was relatively well correlated with the degree of cellular apoptosis and expression pattern of cell death and survival of hEPCs in hypoxia, but, the temporal relationship of the expression pattern of TRx system in HUVECS was less well correlated with the degree of apoptosis, and cell survival signaling in HUVECs during hypoxia. Therefore, the responses of the TRx antioxidative system during hypoxia are slightly different in the two cell types.
There are many kinds of cellular antioxidative systems in the cell membrane, cytosol, and mitochondria. We think that each cell line has peculiar antioxidative capability composed of complex mixture of various kinds of antioxidative systems. Even in similar forms of human EC lines, the TRx system may affect different role and degree of antioxidative capability in hEPC and HUVEC in hypoxic condition. These considerations may give us some solutions for the different apoptotic degree, survival signaling in used 2 cell lines. Up to now, there are many data of the relationship between oxidative stress/antioxidative system and cardiovascular disease, such as hypertension, left ventricular hypertrophy/remodeling, heart failure and atherosclerosis.23)25-28) But, the decisive data for the causal-consequence relationship of those are limited. That problem may be originated from the complexity and non-specificity of oxidative stress and antioxidative system in various disease models.
Our data clearly shows the relationship between the TRx system and apoptosis in ECs during hypoxia. However, the molecular position of the TRx system in the causal-consequence relationship in hypoxia-induced apoptosis is still unknown. To exploit the direct evidence of the impact of the TRx system on hypoxia-induced apoptosis in ECs, further analysis is needed, including using small interfering ribonucleic acid, chemical inhibitos, or an overexpression model of the TRx system of both in vitro cell and in vivo animal models of hypoxia.29) If the role of the TRx system in ECs during hypoxia could be elucidated, it could yield new therapeutic targets for cardiovascular disease involving the TRx system.
Figures and Tables
Fig. 1
Apoptosis in hEPC and HUVEC during hypoxia via FACS analysis. Hypoxia generated by the GasPak system, hEPC showed bimodal peaks of apoptosis at 12 and 24 hours (175.9±35.8%, 398.3±75.8%), 60 and 72 hours (220.5±68.1%, 368.8±20.3%) of hypoxia. HUVECs showed increased apoptosis at 12 hours (256.3±42.2%) of hypoxia initially and the highest degree of apoptosis was observed at 72 hours (500.6±45.6%) of hypoxia. *Significantly different from the control time (p<0.05). hEPC: human endothelial progenitor cells, HUVEC: human umbilical vein endothelial cells, FACS: fluorescence-activated cell sorting.

Fig. 2
The expression pattern of the TRx system in hEPCs and HUVECs during hypoxia. In hEPCs during hypoxia, TRx expression was elevated at 12 and 24 hours (651.3±78.5%, 593.5±64.3%) and thereafter. In HUVECs, TRx expression was increased at 12, 36, and 72 hours of hypoxia (640.1±89.4%, 620.2±98.1%, 595.9±128.4% on each time point), but the over-expression of TRxR and TxNip was not observed during whole hypoxic period. The TRx/TxNip ratio was increased at 36 hours (250.8±77.8%) of hypoxia in hEPCs and at 48 and 72 hours (301.7±49.2%, 550.4±78.4%) of hypoxia in HUVECs. *Significantly different from the control time (p<0.05). TRx: thioredoxin, hEPC: human endothelial progenitor cells, HUVEC: human umbilical vein endothelial cells, TRxR: TRx reductase, TxNip: TRx interacting protein.
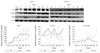
Fig. 3
TRx mRNA expression in hEPCs and HUVECs during hypoxic stress. TRx mRNA expression was increased significantly from 24 hours and thereafter during hypoxia in hEPCs (193.5±56.3%, 188.7±65.9%, 205.9±61.2% on 24, 36, 47 hours), but no increase in TRx mRNA expression in HUVECs was evident during the whole hypoxic period. *Significantly different from the control time (p<0.05). TRx: thioredoxin, mRNA: messenger ribonucleic acid, hEPC: human endothelial progenitor cells, HUVEC: human umbilical vein endothelial cells.

Fig. 4
The expression pattern of Bax and Bcl-2 in hEPCs and HUVECs during hypoxia. In hEPC, Bcl-2 expression showed 2 peaks at 12 and 60 hours (182.7±48.3%, 189.5±52.9%) of hypoxia, Bax expression was not increased significantly. In HUVECs, there were 3 peaks of Bcl-2 expression at 6 to 12, 36, and 60 to 72 hours (259.4±44.9%, 243.4±38.7%, 274.8±46.8%, 220.9±49.7%, 279.3±51.1% on each time point) of hypoxia and the Bax expression pattern was similar. Higher values of the Bax/Bcl-2 ratio were observed at 24 hours (212.4±67.0% vs. 110.8±48.3%) and 60 hours (275.9±86.3% vs. 89.5±59.1%) of hypoxia in hEPCs compared with HUVECs. *Significantly different from the control time (p<0.05), †Significantly different between hEPC and HUVEC (p<0.05). hEPC: human endothelial progenitor cells, HUVEC: human umbilical vein endothelial cells.
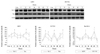
Fig. 5
The expression pattern of Caspase 3, Akt, and Survivin in hEPCs and HUVECs during hypoxia. A steep increase in the expression of Akt was observed in hEPC and HUVECs. Akt overexpression was significantly increased compared with control starting at 12 hours of hypoxia in hEPCs and starting at 24 hours of hypoxia in HUVECs. Caspase 3 and survivin activation was not evident in hEPCs during whole hypoxic period. Caspase 3 overexpression in HUVECs was observed starting at 48 hours (196.8±45.9%) of hypoxia. Higher survivin expression was observed at 12, 60, and 72 hours (385.7±75.2%, 345.6±89.1%, 678.7±95.7% on each time point) of hypoxia in hEPCs. *Significantly different from the control time (p<0.05). hEPC: human endothelial progenitor cells, HUVEC: human umbilical vein endothelial cells, mRNA: messenger ribonucleic acid.
Acknowledgments
This work was supported by a research grant from the Chungbuk National University in 2008.
References
1. Melley DD, Evans TW, Quinlan GJ. Redox regulation of neutrophil apoptosis and the systemic inflammatory response syndrome. Clin Sci. 2005. 108:413–424.
2. Krohn K, Maier J, Paschke R. Mechanism of disease: hydrogen peroxide. DNA damage and mutagenesis in the development of thyroid tumors. Nat Clin Pract Endocrinol Metab. 2007. 3:713–720.
3. Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury: part I. basic mechanisms and in vivo monitoring of ROS. Circulation. 2003. 108:1912–1916.
4. Sorescu D, Weiss D, Lassegue B, et al. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation. 2002. 105:1429–1435.
5. Brasen JH, Leppanen O, Inkala M, et al. Extracellular superoxide dismutase accelerates endothelial recovery and inhibits in-stent restenosis in stented atherosclerotic Watanabe heritable hyperlipidemic rabbit aorta. J Am Coll Cardiol. 2007. 50:2249–2253.
6. Powis G, Briehl M, Oblong J. Redox signaling and the control of cell growth and death. Pharmacol Ther. 1995. 68:149–173.
7. Kang SW, Chae HZ, Seo MS, Kim K, Baines IC, Rhee SG. Mammalian peroxiredoxin isoforms can reduce hydrogen peroxide generated in response to growth factors and tumor necrosis factor-alpha. J Biol Chem. 1998. 273:6297–6302.
8. Nonn L, Williams RR, Erickson RP, Powis G. The absence of mitochondrial thioredoxin 2 causes massive apoptosis, exencephaly, and early embryonic lethality in homozygous mice. Mol Cell Biol. 2003. 23:916–922.
9. Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997. 275:964–967.
10. Masuda H, Kalka C, Asahara T. Endothelial progenitor cells for regeneration. Hum Cell. 2000. 13:153–160.
11. Shioji K, Nakamura H, Masutani H, Yodoi J. Redox regulation by thioredoxin in cardiovascular diseases. Antioxid Redox Signal. 2003. 5:795–802.
12. Haendeler J, Tischler V, Hoffmann J, Zeiher AM, Dimmeler S. Low doses of reactive oxygen species protect endothelial cells from apoptosis by increasing thioredoxin-1 expression. FEBS Lett. 2004. 577:427–433.
13. Kalka C, Masuda H, Takahashi T, et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci U S A. 2000. 97:3422–3427.
14. O'Donnell J, Mille-Baker B, Laffan M. Human umbilical-vein endothelial cells differ from other endothelial cells in failing to express ABO blood group antigens. J Vasc Res. 2000. 37:540–547.
15. Gershoni JM, Palade GE. Electrophoretic transfer of proteins from sodium docedcyl sulfate-polyacrylamide gels to a positively charged mem-brane filter. Anal Biochem. 1982. 124:396–405.
16. Vane JR, Anggard EE, Botting RM. Regulatory functions of the vascular endothelium. N Engl J Med. 1990. 323:27–36.
17. Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events inpatients with coronary artery disease. Circulation. 2001. 104:2673–2678.
18. Fridlyand LE, Philipson LH. Oxidative reactive species in cell injury: mechanisms in diabetes mellitus and therapeutic approaches. Ann N Y Acad Sci. 2005. 1066:136–151.
19. Cho YS, Choi JH, Zhang SY, et al. Relationship of polymorphisms in the oxidative stress related genes-paraoxonase and p22phox to variant angina and coronary artery stenosis in Korean. Korean Circ J. 2003. 33:104–112.
20. Kim KS, Han HS, Lee YS, et al. Plasma thioredoxin level and its correlation to myocardial damage in patients with acute myocardial infarction who underwent successful primary angioplasty. Korean Circ J. 2006. 36:39–45.
21. Ide T, Tsutsui H, Kniganwa S, et al. Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circ Res. 1999. 85:357–363.
22. Powis G, Montfort WR. Properties and biological activities of thioredoxins. Annu Rev Pharmacol Toxicol. 2001. 41:261–295.
23. Becker K, Gromer S, Schirmer RH, Muller S. Thioredoxin reductase as a pathophysiological factor and drug target. Eur J Biochem. 2000. 267:6118–6125.
24. Okuda M, Inoue N, Azumi H, et al. Expression of glutaredoxin in human coronary arteries: its potential role in antioxidant protection against atherosclerosis. Arterioscler Thromb Vasc Biol. 2001. 21:1483–1487.
25. Haendeler J, Hoffmann J, Zeiher AM, Dimmeler S. Antioxidant effects of statins via S-nitrosylation and activation of thioredoxin in endothelial cells: a novel vasculoprotective function of statins. Circulation. 2004. 110:856–861.
26. Nishiyama A, Matsui M, Iwata S, et al. Identification of thioredoxin-binding protein-2/vitamin D(3) upregulated protein 1 as a negative regulator of thioredoxin function and expression. J Biol Chem. 1999. 274:21645–21650.
27. Yoshioka J, Schulze PC, Cupesi M, et al. Thioredoxin-interacting protein controls cardiac hypertrophy through regulation of thioredoxin activity. Circulation. 2004. 109:2581–2586.
28. World CJ, Yamawaki H, Berk BC. Thioredoxin in the cardiovascular system. J Mol Med. 2006. 84:997–1003.
29. Bleeke T, Zhang H, Madamanchi N, Patterson C, Faber JE. Catecholoamine-induced vascular wall growth is dependent on generation of reactive oxygen species. Circ Res. 2004. 94:37–45.
30. Divakaran V, Mann DL. The emerging role of microRNAs in cardiac remodeling and heart failure. Circ Res. 2008. 103:1072–1083.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download