Abstract
Background and Objectives
B-type natriuretic peptide (BNP) or N-terminal pro-BNP (NT-proBNP) levels may serve as a useful marker of cardiovascular risk for screening of the general population. We evaluated reference levels and distribution of NT-proBNP in the Korean general population based on a large cohort study.
Subjects and Methods
We included 1,518 adult subjects (ages 40-69) of a community-based cohort from the Korea Rural Genomic Cohort (KRGC) Study. Thorough biochemical and clinical data were recorded for all subjects. Levels of NT-proBNP from all participants were determined. In order to determine normal reference levels, subjects with factors known to influence NT-proBNP levels were excluded.
Results
The characteristics of the cohort are described below; subjects were 41.2% male, and the mean age was 54.8±8.4 years. The distribution of risk factors for cardiovascular disease in the cohort included hypertension (25%), left ventricular hypertrophy by electrocardiography (ECG-LVH) (15%), hypercholestolemia (4.5%), smoking (32%), diabetes (10.9%), history of coronary heart disease (4.9%), history of heart failure (0.9%), symptoms of heart failure (6.1%), elevated serum creatinine (≥1.5, 3.7%), and severe obesity (body mass index >30 kg/m2, 4.6%). The levels of NT-proBNP of all subjects are shown below; the mean was 60.1±42.1, and the median was 36.5 pg/mL. In addition, the levels of NT-proBNP of normal subjects (which did not have any risk factors, n=224) are shown below; the mean was 40.8, and the median was 32.1 pg/mL. In normal subjects, the NT-proBNP level was slightly higher in females (25.7±24.8 vs. 46.9±35.4, p<0.001). NT-proBNP level increased with age in both the normal population and the total population. There were no significant differences in NT-proBNP levels in subjects who smoked, or had diabetes mellitus, hypertension or ECG-LVH. However, in subjects with a history of congestive heart failure (CHF) (58.5±103.29 vs. 213.8±258.8, p<0.005), elevated serum creatinine levels (≥1.5 mg/dL, 146.2±98.2 vs. 54.3±38.1, p<0.001), or who were older (≥60, 48.4 vs. 84.2±139.5 pg/mL, p<0.05), the BNP level was higher. In addition, patients with more than 3 risk factors for CHF had higher BNP levels (risk 0: 40.8±34.0, 1-2: 57.4±93.2, ≥3: 85.0±152.9 pg/mL). NT-proBNP levels were also related with age, sex, urine albumin, serum Cr, and high sensitivity C-reactive protein (p<0.05).
As cardiac dysfunction increases, the synthesis and release of cardiac natriuretic peptides gradually rises in concert with th other neurohormonal responses observed in heart failure.1) Therefore, it has been proposed that increased B-type natriuretic peptide (BNP) and/or N-terminal pro-BNP (NT-proBNP) be used as a marker for symptomatic ventricular dysfunction.2-5) In addition, irrespective of the degree of left ventricular dysfunction, blood BNP or NT-proBNP levels have been shown to be elevated in patients with many cardiac disorders, including previous myocardial infarction, cardiomyopathy, valvular heart disease, hypertensive heart disease, and atrial fibrillation.6-9) The Framingham study demonstrated that subjects with higher plasma BNP levels exhibited an increased incidence of congestive heart failure (CHF).10) It is therefore possible that BNP or NT-proBNP levels may serve as a useful marker of cardiovascular risk in screening of the general population.11)12) However, recent reports have shown that in the general population, the plasma BNP or NT-proBNP level is affected by many extracardiac factors including age, obesity, and genetics.13-15) However there are few studies on the normal reference levels and distribution of NT-proBNP in the Korean general population.
Therefore, by using our large and well-characterized cohort sample, we sought to establish reference levels of NT-proBNP for healthy individualsand to identify the factors influencing NT-proBNP levels in the general Korean population.
Subjects were selected from the Korean Genomic Rural Cohort, an ongoing epidemiologic study conducted on a representative senior population (age >40 years) of Koreans. Of 3,508 Korean adult subjects 1,437 were men {(mean age; 56.9±7.9) and 2,071 were women (mean age; 55.8±8.1)}. We included 1,518 adult subjects from 3 of 5 total cohort areas for the NT-proBNP assay. Thorough biochemical and clinical data were recorded for all of the participants. Subjects each responded to a questionnaire on their medico-social history and life style characteristics.
In addition, all subjects filled out a heart failure questionnaire about their medical history, symptoms, and drug history. Symptoms of heart failure were determined from questions on ankle swelling and dyspnea grade.16) All subjects provided their informed consent, and the protocol was approved by the Ethics Committee of Yonsei University, Wonju College of Medicine, Wonju, Korea.
We defined "normal subjects" as follows: No history of coronary heart disease (myocardial infarction and/or angina pectoris), stroke, CHF, diabetes mellitus (medical record or chemical examination), renal dysfunction (≥1.5 mg/dL); and no history of cardiovascular symptoms including dyspnea (>grade III), chronic lung disease, chest pain, edema, electrocardiogram (ECG) abnormality, or hypertension (hypertension: systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg, or having a antihypertensive medication history). We included age in evaluating factors that affect NT-proBNP level.
Blood samples were collected to analyze biochemical markers including plasma NT-proBNP, plasma creatinine, and routine chemistry. All blood samples were collected with the participant in a sitting position and after at least 15 minutes of rest. Blood samples for analyses of the biochemical markers were immediately centrifuged at 4℃ and plasma samples were stored in disposable tubes containing aprotinin (a kallikrein inhibitor) in a -80℃ freezer. Plasma concentrations of NT-pro BNP were measured by a novel, highly sensitive and specific immunoassay, based on a sandwich format using unextracted EDTA plasma (Roche). The intra-assay and interassay coefficient of variation for serum in NT-proBNP assays ranged between 2.3% and 5.4%.
For continuous variables, results were expressed as mean±SD. Because the variability of NT-proBNP increased with its mean level, the natural log transformation was used in the regression analyses to satisfy modeling assumptions.
Two sample t-tests were performed to compare the mean or geometric mean values of variables in normal patients, to all other patients. Tests were also performed to compare the mean or geometric mean values of variables between men and women. Pearson's correlation coefficient was used to establish the association between serum NT-proBNP concentrations and clinical and laboratory parameters. Multivariate stepwise logistic regression analyses were performed to identify independent predictors of serum NT-proBNP level. All analyses were done using the Windows-based SPSS statistical package (version 10.0, Chicago, IL, USA), and p<0.05 were considered of significant.
Of 1,518 subjects, 627 were male (41.3%), and the mean age was 54.8±8.4 years. Risk factors for CHF included hypertension (25%), left ventricular hypertrophy (LVH) by ECG (15%), hypercholestolemia (4.5%), smoking (32%), diabetes mellitus (DM) (10.9%), history of coronary heart disease (4.9%), history of CHF (0.9%) heart failure symptoms (6.1%), elevated serum Cr (≥1.5, 3.7%), and obesity {(body mass index (BMI) ≥30, 4.6%} (Fig. 1). Of 1,518 subjects, the 'normal' population having no risk factors was 224 (14.8%, mean age 52.2±6.8), and the population of subjects with risk factors was 1,294 (85.2%, mean age 55.7±9.3).
Plasma NT-proBNP levels were found to range widely, with a distribution skewed lower, towards a normal distribution after log transformation (Fig. 2A). The mean level of NT-pro BNP for all subjects was was 60.1±42.1, the median level was 36.5 pg/mL, and the range was 5-1,425 pg/mL (Fig. 2A). Additionally, the mean level of NT-proBNP for normal subjects was 45.1±86.1, the median level was 31.5 pg/mL, and the range was 5-297 pg/mL. In normal subjects, the level for 97.5% of was 130 pg/mL (Fig. 2B).
In the total population, NT-proBNP levels were not significantly different between men and women (59.6±128.2 vs. 60.4±88.6, p>0.05). However in normal subjects the mean plasma NT-proBNP level was slightly higher in females (25.7±24.8 vs. 46.9±35.4, p<0.001) (Fig. 3). The mean plasma NT-proBNP level increased with age in both groups. The mean plasma NT-proBNP level broke down by age as follows in the total population: for groups 40-49, 50-59, and 60-69, levels were 36.2±49.1, 57.5±102.2 and 84.2±139.4 pg/mL, respectively (p<0.01). We evaluated the mean level of NT-proBNP according to presence of risk factors. There were no significant differences in subjects who smoked, or had DM, hypertension or left ventricular hypertrophy by electrocardiography (ECG-LVH). However, NT-proBNP levels were higher in patients who had a history of CHF (213.8±258.8 vs. 58.5±103.29, p<0.001) (Fig. 4B), a high serum creatinine level (≥1.5 mg/dL, 146.2±98.2 54.3±38.1, p<0.001) or who were older (≥60, 48.4 vs. 84.2±139.5 pg/mL, p<0.005). In subjects with severe obesity (BMI ≥30 kg/m2, 62.4±119.3 vs. 52.1±61.9), NT-proBNP level was lower but the difference was not significant (p=0.068). Fig. 4A shows the correlation between subject's plasma NT-proBNP level and the number of their cardiovascular disease risk factors. These factors include advanced age (≥60 years), hypertension, history of coronary heart disease, mildly elevated serum creatinine level, major ECG abnormalities, smoking history, diabetes mellitus, and obesity. Patients with more than 3 factors had higher NT-proBNP levels (risk 0: 40.8±34.0, 1-2: 57.4±93.2, ≥3: 85.0±152.9 pg/mL, p<0.005) (Fig. 4A).
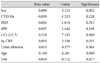
Subjects with plasma NT-proBNP levels ≥97.5 percentile of the gender-age reference level were designated as the high BNP group (n=704). Logistic regression analysis was performed to examine independent clinical factors contributing to high NT-proBNP levels. NT-proBNP levels were related to age, BMI, urine albumin, serum Cr, and high sensitivity C-reactive protein (hs-CRP) (p<0.05). Among these variables, age and high serum creatinine levels were most strongly related to high NT-proBNP levels (Table 1).
In this study, the main findings can be summarized as follows. First, we evaluated the reference levels of plasma NT-proBNP in the Korean adult general population using a large cohort study, and we determined a cut-off value for abnormal NT-proBNP level. Second, we demonstrated NT-proBNP levels depend on physiological conditions including gender, age, and BMI, in addition to pathological risk factors. Finally, we determined that old age, gender, and elevated serum creatinine or hs-CRP levels were significant independent variables associated with high plasma NT-proBNP levels.
Generally, the covariates affecting NT-proBNP concentration were age, sex and BMI.13-15) Some reports have shown that healthy women typically have NT-proBNP concentrations 1.4 times higher than men.16)17) According to our data, in healthy women the NT-proBNP level was 1.6 times higher than men, however in the total population the NT-proBNP level was not different. This may be partially because this group of men has more subjects with elevated creatinine level than the general population. Age is another powerful predictor of increased NT-proBNP levels in normal subjects.18-20) In our study, subjects age 50-59 have a mean NT-proBNP value 1.6 times higher than those age 60-69, and 2.3 times higher than those age 40 to 49. These differences appear to be related to physiological or pathological conditions (such as renal function, LVH, hypertension and CHF). When pathological disease was excluded, slightly higher NT-proBNP values were noted in subjects age 50-59 years compared to those age 40-49. Wang et al.14) reported an inverse relationship between BNP and BMI in a non-obese, healthy subgroup of the Framingham Heart Study offspring cohort, and BMI was an independent negative correlate of BNP in heart failure patients. However, according to our data, while BMI may negatively impact NT-proBNP, the effect is not as impressive or significant as that of age and gender. Ethnicity was also an important factor when considering reference values. It was previously reported that in patients with acute coronary syndromes or acute dyspnea, slightly higher NT-proBNP values were obtained in whites than in blacks.18-20) While though there is no report comparing to East Asian to western people, it is possible there are differences because of ethnicity itself or because of demographic and physiological variables. Establishing normal values is essential to the interpretation of results for an individual patient. Not all of the subjects who were considered "normal" in our cohort study were completely healthy. While we evaluated detailed patient history, some of the patients might have asymptomatic cardiac dysfunction or other noncardiac conditions. Because complete cardiac evaluation was not performed, it is theoretically possible that a small minority of the subjects had altered NT-proBNP levels due to their conditions.21)
Smoking, DM, hypertension, and ECG-LVH have been reported to affect NT-proBNP levels.22-25) However in our data they appear to have no affect. Because we did not evaluate precise left ventricular function and hypertrophy by echocardiography, we do not know if there was left ventricular hypertrophy or dysfunction in subjects with DM, hypertension, or ECG-LVH. We would have needed to perform morphological analysis of the left ventricle. Usually, both BNP and the amino-terminal fragment of its pro-hormone (NT-proBNP) are elevated in patients with renal dysfunction and end-stage renal disease.26)27) However the relationship between renal function, circulating NT-proBNP have not been fully elucidated. We found no independent relationship between serum creatinine and NT-proBNP in normal patients. However in the total population, subjects with high serum creatinine level had high NT-proBNP levels, and high serum creatinine level was the most strong correlated factor with high NT-proBNP levels.
Some have reported that renal impact is minor if the degree of dysfunction is small.26) However our data showed interdependence between renal impairment and elevated NT-proBNP.27) This may be because NT-proBNP does not have a clearance receptor, so its clearance may be influenced by renal function.28)
When considering the application of cutoff points, it is always necessary to acknowledge the population to which they apply;29) thus, cutoff points derived for a healthy population might not necessarily apply to an acutely ill population or a hospitalized population. Plasma NT-proBNP levels may be elevated in subjects with precursors of CHF, and thus may serve as a useful predictor for the new onset of CHF. They might also be useful for high risk recognition in a screening setting in the general population.10-11)30)
To the best of our knowledge, this is the first study to provide reference plasma NT-proBNP values in a Korean community-based population. We suggest that this reference value will serve as a useful reference for NT-proBNP for screening of general population. As Framingham and others have reported, BNP or NT-proBNP is a well known risk factor for CHF or cardiovascular death.10)31) It is important to identify subjects at high risk for CHF with asymptomatic heart disease who will be prone to develop overt CHF. The present study demonstrates high plasma BNP levels may serve as a marker for subjects with an increased risk of CHF.
Taken together, adjustments for the independent effects of age and sex appear necessary in considering the reference value for normal plasma NT-proBNP. In addition, we have identified many other confounding variables involved in the interpretation of a given plasma NT-proBNP concentration, and of these impaired renal function seems to be the most important.
Our study has several limitations. The first is selection bias. It is possible there may be differences between this regional cohort and data from a real demographic background in Korea. In our study, we excluded subjects greater than age 70 because of the original cohort design. Second, although we obtained the reference level after careful history evaluation and examination for hypertension, ECG abnormality, previous cardiovascular disease, cardiovascular medications, and cardiac symptoms, we did not performed echocardiography in all participations. We are performing an ongoing study involving echocardiography in same cohort. Finally, analytical problems were possible in our cohort study. Although NT-pro BNP was more stable than BNP,32) there are always possible effects from sample storageand collection. These issues must be considered when examining our reference levels.
Figures and Tables
Fig. 1
Prevalence of risk factors for congestive heart failure in cohort subjects. CHD: coronary heart disease, HF: heart failure, BMI: body mass index, DM: diabetes mellitus, LVH: left ventricular hypertrophy, ECG: electrocardiogram, s-Cr: serum creatinine.

Fig. 2
The distribution of NT-proBNP levels from cohort study. A: total population. B: normal population. NT-proBNP: N-terminal pro-B-type natriuretic peptide.

Fig. 3
The effect of gender on NT-proBNP levels in the normal population and total population. Data represent mean±SE. *p<0.05, †p<0.005 among different groups in the same population. NT-proBNP: N-terminal pro-B-type natriuretic peptide. SE: standard error.

Fig. 4
The effect of risk factors (A) and presence of CHF (B) on NT-pro BNP levels. Data represent mean±SE. *p<0.05, †p<0.005 among different groups in the same population. CHF: congested heart failure, NT-proBNP: N-terminal pro-B-type natriuretic peptide, SE: standard error.

Acknowledgments
This study was supported by a grant of The Korean Society of Circulation in 2006 (supported by GSK-Korea, to Byung-Su Yoo) and the present work was supported in part by Roche diagnostics-Korea and AstraZeneca-Korea.
References
1. Tsutamoto T, Wada A, Maeda K, et al. Attenuation of compensation of endogenous cardiac natriuretic peptide system in chronic heart failure: prognostic role of plasma brain natriuretic peptide concentration in patients with chronic symptomatic left ventricular dysfunction. Circulation. 1997. 96:509–516.
2. Cowie MR, Struthers AD, Wood DA, et al. Value of natriuretic peptides in assessment of patients with possible new heart failure in primary care. Lancet. 1997. 350:1349–1353.
3. Dao Q, Krishnaswamy P, Kazanegra R, et al. Utility of B-type natriuretic peptide in the diagnosis of congestive heart failure in an urgent-care setting. J Am Coll Cardiol. 2001. 37:379–385.
4. Maisel AS, Krishnaswamy P, Nowak RM, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002. 347:161–167.
5. Yoo BS, Kim WJ, Jung HS, et al. The clinical experiences of B-type natriuretic peptide blood concentrations for diagnosis in congestive heart failure: the single hospital experience based on the large clinical database. Korean Circ J. 2004. 34:684–692.
6. Sutton TM, Stewart RA, Gerber IL, et al. Plasma natriuretic peptide levels increase with symptoms and severity of mitral regurgitation. J Am Coll Cardiol. 2003. 41:2280–2287.
7. Kohno M, Horio T, Yokokawa K, et al. Brain natriuretic peptide as a cardiac hormone in essential hypertension. Am J Med. 1992. 92:29–34.
8. Rossi A, Enriquez-Sarano M, Burnett JC Jr, Lerman A, Abel MD, Seward JB. Natriuretic peptide levels in atrial fibrillation: a prospective hormonal and Doppler-echocardiographic study. J Am Coll Cardiol. 2000. 35:1256–1262.
9. Choe H, Yoo BS, Ryu HY, et al. The early changing pattern of the B-type natriuretic peptide concentration and its significance as a prognostic marker after acute myocardial infarction. Korean Circ J. 2006. 36:526–534.
10. Wang TJ, Larson MG, Levy D, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004. 350:655–663.
11. Nakamura M, Endo H, Nasu M, Arakawa N, Segawa T, Hiramori K. Value of plasma B type natriuretic peptide measurement for heart disease screening in a Japanese population. Heart. 2002. 87:131–135.
12. McDonagh TA, McDonald K, Maisel AS. Screening for asymptomatic left ventricular dysfunction using B-type natriuretic Peptide. Congest Heart Fail. 2008. 14:4 Suppl 1. 5–8.
13. Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC Jr. Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol. 2002. 40:976–982.
14. Wang TJ, Larson MG, Levy D, et al. Impact of obesity on plasma natriuretic peptide levels. Circulation. 2004. 109:594–600.
15. Wang TJ, Larson MG, Levy D, et al. Heritability and genetic linkage of plasma natriuretic peptide levels. Circulation. 2003. 108:13–16.
16. Raymond I, Groenning BA, Hildebrandt PR, et al. The influence of age, sex and other variables on the plasma level of N-terminal pro brain natriuretic peptide in a large sample of the general population. Heart. 2003. 89:745–751.
17. Segawa T, Nakamura M, Itai K, Onoda T, Okayama A, Hiramori K. Plasma B-type natriuretic peptide levels and risk factors for congestive heart failure in a Japanese general population. Int Heart J. 2005. 46:465–475.
18. Ordonez-Llanos J, Collinson PO, Christenson RH. Amino-terminal pro-B-type natriuretic peptide: analytic considerations. Am J Cardiol. 2008. 101:9–15.
19. Krauser DG, Chen AA, Tung R, Anwaruddin S, Baggish AL, Januzzi JL Jr. Neither race nor gender influences the usefulness of aminoterminal pro-brain natriuretic peptide testing in dyspneic subjects: a ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) substudy. J Card Fail. 2006. 12:452–457.
20. Januzzi JL Jr, Chen-Tournoux AA, Moe G. Amino-terminal pro-B-type natriuretic peptide testing for the diagnosis or exclusion of heart failure in patients with acute symptoms. Am J Cardiol. 2008. 101:29–38.
21. Galasko GI, Lahiri A, Barnes SC, Collinson P, Senior R. What is the normal range for N-terminal pro-brain natriuretic peptide?: how well does this normal range screen for cardiovascular disease? Eur Heart J. 2005. 26:2269–2276.
22. März W, Tiran B, Seelhorst U, et al. N-terminal pro-B-type natriuretic peptide predicts total and cardiovascular mortality in individuals with or without stable coronary artery disease: the Ludwigshafen Risk and Cardiovascular Health Study. Clin Chem. 2007. 53:1075–1083.
23. Magnusson M, Melander O, Israelsson B, Grubb A, Groop L, Jovinge S. Elevated plasma levels of Nt-proBNP in patients with type 2 diabetes without overt cardiovascular disease. Diabetes Care. 2004. 27:1929–1935.
24. Hildebrandt P, Richards AM. Amino-terminal pro-B-type natriuretic peptide testing in patients with diabetes mellitus and with systemic hypertension. Am J Cardiol. 2008. 101:21–24.
25. Goetze JP, Mogelvang R, Maage L, et al. Plasma pro-B-type natriuretic peptide in the general population: screening for left ventricular hypertrophy and systolic dysfunction. Eur Heart J. 2006. 27:3004–3010.
26. Bruch C, Fischer C, Sindermann J, Stypmann J, Breithardt G, Gradaus R. Comparison of the prognostic usefulness of N-terminal pro-brain natriuretic peptide in patients with heart failure with versus without chronic kidney disease. Am J Cardiol. 2008. 102:469–474.
27. Wang HS, Yoo BS, Chung IY, et al. Is B-type natriuretic peptide (BNP) measurement useful test for diagnosing systolic heart failure in patients with moderate to severe renal insufficiency? Korean Circ J. 2005. 35:897–903.
28. Almirez R, Protter AA. Clearance of human brain natriuretic peptide in rabbits: effect of the kidney, the natriuretic peptide clearance receptor, and peptidase activity. J Pharmacol Exp Ther. 1999. 289:976–980.
29. Coste J, Jourdain P, Pouchot J. A gray zone assigned to inconclusive results of quantitative diagnostic tests: application to the use of brain natriuretic peptide for diagnosis of heart failure in acute dyspneic patients. Clin Chem. 2006. 52:2229–2235.
30. Ewald B, Ewald D, Thakkinstian A, Attia J. Meta-analysis of B type natriuretic peptide and N-terminal pro B natriuretic peptide in the diagnosis of clinical heart failure and population screening for left ventricular systolic dysfunction. Intern Med J. 2008. 38:101–113.
31. Austin MJ, Heneghan MA. Multiple biomarkers and cardiovascular risk. N Engl J Med. 2008. 359:760–761.
32. Mueller T, Gegenhuber A, Dieplinger B, Poelz W, Haltmayer M. Long-term stability of endogenous B-type natriuretic peptide (BNP) and amino terminal proBNP (NT-proBNP) in frozen plasma samples. Clin Chem Lab Med. 2004. 42:942–944.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download