Abstract
Overweight and obesity are rapidly increasing in prevalence due to adoption of the westernized life style in Korea. Obesity is strongly associated with the development of cardiovascular risk factors such as diabetes, hypertension, and dyslipidemia. In addition, accumulating evidence suggests that obesity per se has a direct effect on cardiac functional and structural changes that may not be the result of atherosclerosis. In this review, we focus on the view that obesity can influence on the structural and functional changes of the heart, drawing evidence from human and animal studies. We also review influencing factors such as physical, neurohormonal, and metabolic alterations that are associated with changes of the heart in obesity.
The prevalence of obesity is increasing due to the increasing adoption of westernized life styles in Korea. Obesity is defined by body mass index (BMI), which is classified as underweight (<20 kg/m2, normal (20-24.9 kg/m2, overweight (25-29.9 kg/m2, and obese (≥30 kg/m2. Korean National Health Examination and Nutrition Survey data in 2001 estimated that 3.0% of Korean adults were obese and 29.5% were overweight.1) The prevalence of overweight Korean children and adolescents doubled from 5.4% in 1998 to 11.4% in 2001.2) Increasing degrees of obesity are closely correlated with the increasing rates of cardiovascular disease.3) Furthermore, a large body of evidence strongly supports the view that obesity itself is associated with preclinical structural and functional changes in the heart, which prelude heart failure.4) In this review, we discuss the changes of cardiac structure and function in obesity and focus on the current evidence regarding the causative role of obesity in cardiac changes.
Two types of animal models are used in obesity studies: genetic mutant models and high fat diet-induced obesity model. Genetic mutant mice models, such as ob/ob and db/db, present severe types of obesity. The ob/ob and db/db mice models become progressively obese, left ventricular (LV) hypertrophy develops, and LV mass increases. Interestingly, leptin infusion to these mice leads to decreased myocardial wall thickness.5) LV hypertrophy is a common cardiac phenotype in the response of genetic mutant mice to obesity. Use of high fat diet mice has demonstrated that increased non-esterified fatty acid (NEFA) contents, which diminish the rate of glucose metabolism and increase oxygen consumption, result in reduced ability to recover from a workload.6) Furthermore, high fat diet mice also display elevated blood pressure and impaired insulin receptor activation.7) All of these factors contribute to the development of cardiac hypertrophy and LV dysfunction in high fat diet mice.7)
Results from the two animal models indicate that obesity may result in cardiac hypertrophy due to insulin resistance (IR), metabolism alteration such as leptin deficiency, or leptin resistance and elevated blood pressure.
Obesity is an independent factor for LV hypertrophy.8-11) Obesity itself may have hemodynamic effects that produce an increase in the total blood volume and cardiac output due to the high metabolic activity of excessive fat. In moderate to severe obesity, these increases may lead to LV dilation, increased LV wall stress, and compensatory (eccentric) LV hypertrophy.8)9) Despite this logical explanation, recent studies have shown that concentric LV hypertrophy is a predominant form in obese subjects.10)11) Besides hemodynamic factors, hyperinsulinemia due to IR, which stimulates insulin like growth factor-1 (IGF-1) receptors, is also involved in the pathogenesis of LV hypertrophy.12) IGF-1 enhances anabolic effects on the myocardium, and facilitates increased myocardial mass and concentric hypertrophy. Systolic hypertension is traditionally known as a major contributor to LV hypertrophy rather than obesity. However, in the Framingham heart study, the investigators found independent influences of BMI and blood pressure on LV mass index.13) Therefore, the effects of obesity and blood pressure were additive on LV changes. However, opinion is divided concerning which components are the stronger predictor of LV hypertrophy. Obesity was the strongest clinical predictor for LV hypertrophy in several studies,12)14) while another study showed that BMI and hypertension affect LV hypertrophy similarly.15)
In summary, obesity induces LV hypertrophy via hemodynamic changes caused by hyperinsulinemia, increased IGF-1 expression, and volume overload, which are similar to the effect caused by hypertension.
Epicardial fat mass, as determined by echocardiographic and magnetic resonance imaging studies, may reflect intra-abdominal visceral fat. Therefore, epicardial fat mass could serve as a marker of visceral adiposity.16) The relationship between local adipose tissue in heart and cardiac geometry has recently been studied. Increased epicardial fat mass and fatty infiltration of myocardium contributed to the increased cardiac mass.17) Although epicardial fat is normally accounted for about 20% of the total ventricular mass, total epicardial fat weights are significantly greater in hypertrophic heart.18)
The causal effect of epicardial adipose tissue on cardiac remodeling remains unsolved. However, epicardial adipose tissue serves as an endocrine organ, which releases monocyte chemotactic protein-1, interleukin-1β, interleukin-6 and tumor necrosis factor-alpha. These adipokines may be important factors in cardiac remodeling or hypertrophy in obese subjects.19)
Left atrial (LA) size and LA volume is increased in the setting of obesity compared with non-obese individuals.20) The possible mechanisms of increased LA size and volume in obesity are similar to those of LV hypertrophy in obesity; increased BMI leads to volume overload,21) which causes diastolic abnormality of LV filling defect. Diastolic dysfunction can contribute to LA enlargement or remodeling.22) In the Framingham heart study, obesity was found to be a strong risk factor for development of atrial fibrillation even after accounting for concomitant conditions such as hypertension, diabetes mellitus, and myocardial infaction.23) In the clinical setting, dilated LA size in the absence of organic heart disease or atrial fibrillation is considered to be a risk factor for developing atrial fibrillation and long-term cardiovascular events.24)
The effects of obesity on LV systolic function are controversial. Several studies reported that LV systolic function is normal or increased in obesity.8)9)25) However, some evidence from non-invasive or invasive techniques suggests that obesity causes a subclinical contractility abnormality.11)26)27) Obesity-induced myocardial dysfunction can be explained by the derangement of myocardial metabolism. In animal models, IR in obesity cause alterations in myocardial fatty acid metabolism and efficiency (cardiac work/myocardial oxygen consumption) that occur early in the cascade of events, leading to impaired LV contractility.28) In human studies, obesity is a significant predictor of increased myocardial oxygen consumption and decreased efficiency, and IR is a robust predictor of fatty acid uptake, utilization, and oxidation. These metabolic changes may play a role in the pathogenesis of decreased cardiac performance in obesity.29) Collectively, obesity might be a strong factor that can induce LV systolic dysfunction and eventually cause heart failure independent to coronary artery disease or other morbidities.
Echocardiography studies conducted with obese animals and human have provided inconsistent results about E-wave velocity, deceleration time, A-wave velocity.11)30) Prolongation of the isovolumic relaxation time may be the most consistent diastolic abnormality in obesity.31)32) In uncomplicated obese subjects, diastolic dysfunction is caused by hemodynamic and metabolic factors. Hemodynamic changes cause diastolic dysfunction in obese subjects through LV hypertrophy.33) Metabolic factors will be discussed later chapter. Other potential mediators include hormones and cytokines released in association with obesity. Changes of adipokine concentration in serum, such as leptin and adiponectin, are observed in obese subjects, which may also be partly responsible for the LV diastolic dysfunction. Studies with ob/ob mice (which are deficient in leptin) have demonstrated diastolic dysfunction by the changes of myocardial fatty acid and glucose metabolsim.28)34) Similarly, leptin resistance and hyperleptinemia are observed in obese subjects, which may lead to diastolic dysfunction. Circulating total and high-molecular-weight adiponectin are negatively correlated with LV wall thickness and diastolic dysfunction independent of age and metabolic factors.35) In the Otsuka Long-Evans Tokushima Fatty rat model of pre-diabetes, pre-diabetic conditions cause an accumulation of myocardial collagen, leading to interstitial and perivascular fibrosis, which correlates with LV early diastolic dysfunction.36)
Collectively, the evidence indicates that the preclinical diastolic dysfunction of obesity is related to hemodynamic alteration, various adipokines, and myocardial collagen accumulation.
Obstructive sleep apnea (OSA) is a very common abnormality in obese subjects. The Wisconsin Sleep Cohort Study demonstrated that the risk of development of hypertension in OSA patients is three times higher than non-OSA subjects.39) Likewise, OSA patients demonstrated not only day-time hypertension but night-time hypertension or non-dipper pattern.39) Repetitive episodes of airway obstruction cause hypoxemia and changes of intra-thoracic pressure, which in turn causes sympathetic overactivity. Furthermore, chronic hypoxemia causes injury to myocytes and cardiac extracellular matrix. All of these factors-hypertension, sympathetic overactivity, hypoxemia-lead to ventricular remodeling.40) One study reported that 88% of OSA patients have LV hypertrophy and 64% of those have LA enlargement.41) The authors also demonstrated that the use of continuous positive airway pressure for 6 months could reduce LV hypertrophy.41) All these findings suggest that the severity of nocturnal hypoxemia could be important in the development of LV hypertrophy in obese subjects with OSA.
Energy sources of myocardial metabolism are different from diabetes compared to normal glucose tolerance subjects. In the setting of diabetes or IR, myocardium utilizes much more free-fatty acid and less glucose, which occurs even in the early course of obesity.42) Long-standing caloric excess or obesity activates peroxisome proliferator-activated receptor-α/peroxisome proliferator-activated receptor-γ coactivator signaling, which increases the expression of genes involved in fatty acid oxidation and fatty acid transporters such as FATP1 and CD36.43)44) In human, obesity is also associated with increased rates of fatty acid oxidation, increased myocardial oxygen consumption, and reduced cardiac performance proportionate to the degree of IR and obesity.29) Indeed, normalization of cardiac metabolism by overexpression of a human GLUT4 transgene in db/db mice reverses cardiac dysfunction, which suggests that altered myocardial metabolism contributes to contractile dysfunction in this model.45) It has been demonstrated that mitochondrial oxygen consumption rate and adenosine triphosphate (ATP) generation capacity are reduced in ob/ob mice, and that the protein levels of mitochondrial complexes I, III, V for the oxidative phosphorylation are significantly reduced in db/db mice.46) Because of mitochondrial response in obesity or diabetes, the ratio of ATP generation and oxygen consumption is reduced (mitochondrial uncoupling), which is believed to be a significant factor for declination of cardiac function.46) Mitochondria also represent a major source of superoxide production. In db/db mice, mitochondrial reactive oxygen species generation increases, which can damage myocardial cells.46)
In summary, cardiac metabolic changes, mitochondrial dysfunction, and oxidative stress can cause declination of cardiac function in obese subjects.
IR represents problems of insulin receptor, insulin signaling, and genetics. Among these problems, insulin signaling impairment could be the key factor for IR. Impaired insulin-mediated activation of intracellular signaling has been described in ob/ob mice.42) In the animal models, obesity and IR can increase myocardial fatty acid uptake, which causes myocardial fatty acid oxidation and myocardial oxygen consumption. Persistence of this metabolic change causes an imbalance of fatty acid uptake and fatty acid oxidation, leading to accumulation of fatty acid intermediates and ceramide production, which impairs myocardial function and increases apoptosis of myocardocytes.47)48) This phenomenon has also been demonstrated in human.29) However, in Zucker fatty rats treated with the insulin sensitizer thiazolinedione (TZD), myocardial glucose consumption was increased, fatty acid oxidation was diminished, and myocardial injury was reduced.49) In addition, hyperinsulinemia stimulates IGF-1 receptors, which is likely responsible in the pathogenesis of myocardial hypertrophy.12)
In general, obese subjects have activated sympathetic tone. This leads to concentric LV hypertrophy due to elevated afterload and increased cardiac contractility. Additionally, catecholamine directly affects the myocardium without hemodynamic influence. The renin-angiotensin system also affects the heart via hemodynamic effects or direct signaling in obesity.50) Angiotensinogen secretion and its messenger ribonucleic acid (mRNA) have been detected in fa/fa rat adipocytes and in human.51) Angiotensin is thought to be a contributor to the sympathoexcitation, therefore the rennin-angiotensin system could increase central sympathetic activation and have a role as an additive effect to blood pressure elevation and cardiac remodeling in obese subjects.52)
The compositional changes of the extracellular matrix is an important contributor in cardiac remodeling.53) Serum levels of cardiac collagen synthesis have been significantly associated with IR in normotensive and non-diabetic obese subjects.54) In another study using a rabbit model of obesity, a high-fat diet caused fibrosis in coronary vessels as well as the accumulation of collagen in the cardiac interstitium.55)
Adipokines such as adiponectin or leptin play an important role in the extracellular matrix changes of the heart. Leptin is an adipokine that is produced in the obese gene (ob) located in adipocytes.56) In one study, leptin increased matrix metalloproteinase-2 (MMP-2) secretion and its mRNA expression, and attenuated collagen I mRNA synthesis and increased collagen III and IV, but did not change total collagen synthesis in human pediatric ventricular myocytes.57) In a diet-induced obesity murine model, elevated leptin level was detected after 20 weeks of a high fat diet, and this leptin level was correlated with reduced ventricular shortening and increased LV posterior wall thickness.58) In the coronary ligation model, procollagen I and III levels were elevated after 7 days postinfarction. Furthermore when the neutralizing leptin antibody was injected, enhanced collagen was attenuated.59)
Adiponectin is considered a cardioprotective adipokine. A recent study suggested that adiponectin level showed positive correlation with tissue inhibitor of metalloproteinase (TIMP), which is considered to exert an antifibrotic effect.60) The adiponetin levels are decreased in the obese or IR subjects; the cardioprotective effect of adiponectin, especially its antifibrotic effect, is diminished in obese subjects.
In obese subjects, adipocytokines such as catecholamine, rennin-angiotensin-aldosterone system, tumor necrosis factor-alpha, C-reactive protein, leptin, and adiponectin influence to MMP activity, TIMP expression, and collagen synthesis, which ultimately leads to cardiac remodeling.53)
The evidence of cardiac apoptosis in the genesis of heart failure has surged over the last decade. Apoptotic cardiomyocyte death has been proven from biopsies of dilated cardiomyopathy and end stage heart failure.61) Besides apoptotic cell death, activation of several proteases in the apoptotic cascade can cleave contractile proteins including actin, myosin, and troponins.62) There are numerous causes of apoptosis. Apoptosis appears to be ischemia or ischemia-reperfusion driven at the site of infarct and at sites remote from the ischemic area through neurohormonal effects. Myocardial stretch, wall stress, cytokines, and neurohormones such as norepinephrine and angiotensin II, which are commonly produced as a part of peripheral neurohormonal rearrangements after an acute myocardial infarction, have been demonstrated to enhance apoptosis.63)64)
Studies for obesity associated cardiac apoptosis are lacking. Use of the Zucker fatty rat model has shown that cardiac contractility is reduced with increased cardiac cell apoptosis; however, when the rats were treated with TZD, cardiac cell apoptosis was decreased.48) Another study involving a high fat diet induced IR rat model did not reveal a significant difference in apoptosis compared with control group, despite impaired cardiac function.65) Therefore, it is necessary to study the relationship between obesity, cardiac cell apoptosis, and impairment of cardiac function.
Weight reduction improves systolic blood pressure, IR, sleep apnea, and hyperlipidemia. Weight reduction also has beneficial effects on cardiac structure and function such as reduction of LV diameter, LV wall thickness, LV mass, and LA dimension.66) However, removal of subcutaneous fat by liposuction does not produce a beneficial effect on metabolic changes, so it has little effect on the cardiovascular system.67) Pharmacological-mediated weight reduction is recommended for patients in whom lifestyle modification has failed. Oristat, which is a gastrointestinal lipase inhibitor, can reduce weight by about 3 kg and decreases progression to diabetes in high risk patients.68) Sibutramine, a monoamine reuptake inhibitor, can reduce weight by about 4-5 kg and improves serum lipid levels and insulin sensitivity,69) but it increases heart rate and blood pressure. Rimonabant, an endocannabinoid receptor blocker, reduces weight by 4-5 kg, decreases waist circumference, and improves serum high density lipoprotein cholesterol and triglyceride levels.70) However, there are no definitive data for the effect on cardiac structure and function in use of these medications.71) Therefore, weight reduction by diet and exercise is still the most important means to correct the functional and structural deteriorations that occur in the heart in obese individuals.
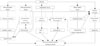
Obesity is a causative factor for development of preclinical changes of the heart. In obese subjects, initially volume overload develops, which leads to increased cardiac output and hypertension. Second, sympathetic and rennin-angiotensin-aldosterone system activity is increased, which causes hypertension. Third, hyperinsulinemia (IR) increases advanced glycation end-products and IGF-1 production and facilitates fatty acid uptake, which leads to lipotoxic cell damage. Fourth, visceral adipose tissue affects oxidative stress and is related with decreased leptin level or leptin resistance and decreased adiponectin level; these changes affect cellular changes and metabolic alteration. Fifth, cellular changes are produced by neurohormonal and metabolic factors, oxidative stress, and adipokines, which leads to extracellular fibrosis and apoptosis. All these descriptive factors eventually lead to structural and functional changes of the heart in obesity (Fig. 1). Therapeutically, life-style associated weight reduction may be more important than any other molecular- or pathway-targeted therapy.
Figures and Tables
References
1. Park HS, Park CY, Oh SW, Yoo HJ. Prevalence of obesity and metabolic syndrome in Korean adults. Obes Rev. 2008. 9:104–107.
2. Kim HM, Park J, Kim HS, Kim DH, Park SH. Obesity and cardiovascular risk factor in Korean children and adolescents aged 10-18 years from the Korean National Health and Nutrition Examination Survey, 1998 and 2001. Am J Epidemiol. 2006. 164:787–793.
3. Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983. 67:968–977.
4. Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med. 2002. 347:305–313.
5. Barouch LA, Berkowitz DE, Harrison RW, O'Donnell CP, Hare JM. Disruption of leptin signaling contributes to cardiac hypertrophy independently of body weight in mice. Circulation. 2003. 108:754–759.
6. Korvald C, Elvenes OP, Myrmel T. Myocardial substrate metabolism influence left ventricular energetic in vivo. Am J Physiol Heart Circ Physiol. 2000. 278:H1345–H1351.
7. Ouwens DM, Boer C, Fodor M, et al. Cardiac dysfunction induced by high -fat diet is associated with altered myocardial insulin signaling in rats. Diabetologia. 2005. 48:1229–1237.
8. Avelar E, Cloward TV, Walker JM, et al. Left ventricular hypertrophy in severe obesity: interaction among blood pressure, nocturnal hypoxemia, body mass. Hypertension. 2007. 49:34–39.
9. Wong CY, O'Moore-Sullivan T, Leano R, Byrne N, Beller E, Marwick TH. Alterations of the left ventricular myocardial characteristics associated with obesity. Circulation. 2004. 110:3081–3087.
10. Iacobellis G, Ribaudo MC, Zappaterreno A, Iannucci CV, Di Mario U, Leonetti F. Adapted changes in left ventricular structure and function in severe uncomplicated obesity. Obes Res. 2004. 12:1616–1621.
11. Peterson LR, Waggoner AD, Schechtman KB, et al. Alterations in left ventricular structure and function in young healthy obese women: assessment by echocardiography and tissue Doppler imaging. J Am Coll Cardiol. 2004. 43:1399–1404.
12. Sundstrom J, Lind L, Valind S, et al. Myocardial insulin-mediated glucose uptake and left ventricular geometry. Blood Press. 2001. 10:27–32.
13. Lauer MS, Anderson KM, Levy D. Separate and joint influence of obesity and mild hypertension on left ventricular mass and geometry: the Framingham Heart Study. J Am Coll Cardiol. 1992. 19:130–134.
14. Gottdiener JS, Reda DJ, Materson BJ, et al. Importance of obesity, race and age to the cardiac structure and functional effect of hypertension. J Am Coll Cardiol. 1994. 24:1492–1498.
15. Fox E, Taylor H, Andrew M, et al. Body mass index and blood pressure influence on left ventricular mass and geometry in African Americans: the Atherosclerotic Risk In Communities (ARIC) Study . Hypertension. 2004. 44:55–60.
16. Iacobellis G, Leonetti F. Epicardial adipose tissue and insulin resistance in obese subjects. J Clin Endocrinol Metab. 2005. 90:6300–6302.
17. Iacobellis G, Ribaudo MC, Zappaterreno A, Iannucci CV, Leonetti F. Relation between epicardial adipose tissue and left ventricular mass. Am J Cardiol. 2004. 94:1084–1087.
18. Corradi D, Maestri R, Callegari S, et al. The ventricular epicardial fat is related to the myocardial mass in normal, ischemic and hypertrophic hearts. Cardiovasc Pathol. 2004. 13:313–316.
19. Malavazos AE, Ermetici F, Coman C, Corsi MM, Morricone L, Ambrosi B. Influence of epicardial adipose tissue and adipocytokine levels on cardiac abnormalities in visceral obesity. Int J Cardiol. 2007. 121:132–134.
20. Ayer JG, Almafragy HS, Patel AA, Hellyer RL, Celermajer DS. Body mass index is an independent determinant of left atrial size. Heart Lung Circ. 2008. 17:19–24.
21. Di Bello V, Santini F, Di Cori A, et al. Obesity cardiomyopathy: is it a reality? An ultrasonic tissue characterization study. J Am Soc Echocardiogr. 2006. 19:1063–1071.
22. Pritchett AM, Mahoney DW, Jacobsen SJ, Rodeheffer RJ, Karon BL, Redfield MM. Diastolic dysfunction and left atrial volume: a population-based study. J Am Coll Cardiol. 2005. 45:87–92.
23. Wang TJ, Parise H, Levy D, et al. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004. 292:2471–2477.
24. Tsang TS, Barnes ME, Miyasaka Y, et al. Obesity as a risk factor for the pregression of paroxysmal to permanent atrial fibrillation: a longitudinal cohort study of 21 years. Eur Heart J. 2008. 29:2227–2233.
25. Messerli FH, Ventura HO, Reisin E, et al. Borderline hypertension and obesity: two prehypertensive states with elevated cardiac output. Circulation. 1982. 66:55–60.
26. Aurigemma GP, Silver KH, Priest MA, Gaasch WH. Geometric changes allow normal ejection fraction despite depressed myocardial myocardial shortening in hypertensive left ventricular hypertrophy. J Am Coll Cardiol. 1995. 26:195–202.
27. Garavaglia GE, Messerli FH, Nunez BD, Schmieder RE, Grossman E. Myocardial contractility and left ventricular function in obese patients with essential hypertension. Am J Cardiol. 1988. 62:594–597.
28. Aasum E, Hafstad AD, Severson DL, Larsen TS. Age-dependent changes in metabolism, contractile function, and ischemic sensitivity in hearts from db/db mice. Diabetes. 2003. 52:434–441.
29. Peterson LR, Herrero P, Schechtman KB, et al. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation. 2004. 109:2191–2196.
30. Pascual M, Pascual DA, Soria F, et al. Effect of isolated obesity on systolic and diastolic left ventricular function. Heart. 2003. 89:1152–1156.
31. Berkalp B, Cesur V, Corapcioglu D, Erol C, Baskal N. Obesity and left ventricular diastolic dysfunction. Int J Cardiol. 1995. 52:23–26.
32. Morricone L, Malavazos AE, Coman C, Donati C, Hassen T, Caviezel F. Echocardiographic abnormalities in normotensive obese patients: relationship with visceral fat. Obes Res. 2002. 10:489–498.
33. Chakko S, Mayor M, Allison MD, Kessler KM, Materson BJ, Myerburg RJ. Abnormal left ventricular diastolic filling in eccentric left ventricular hypertrophy of obesity. Am J Cardiol. 1991. 68:95–98.
34. Christoffersen C, Bollano E, Lindegaard ML, et al. Cardiac lipid accumulation associated with diastolic dysfunction in obese mice. Endocrinology. 2003. 144:3483–3490.
35. Kozakova M, Muscelli E, Flyvbjerg A, et al. Adiponectin and left ventricular structure and function in healthy adults. J Clin Endocrinol Metab. 2008. 93:2811–2818.
36. Mizushige K, Yao L, Noma T, et al. Alteration in left ventricular diastolic filling and accumulation of myocardial collagen at insulin-resistant prediabetic stage of a type II diabetic rat model. Circulation. 2000. 101:899–907.
37. Messerli FH, Christie B, DeCarvalho JGR, et al. Obesity and essential hypertension; hemodynamics, intravascular volume, sodium excretion, and plasma renin activity. Arch Intern Med. 1981. 141:81–85.
38. Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999. 282:1523–1529.
39. Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000. 342:1378–1384.
40. Quan SF, Gersh BJ. Cardiovascular consequences of sleep-disordered breathing: past, present and future: report of a workshop from the National Center on Sleep Disorders Research and the National Heart, Lung, Blood Institute. Circulation. 2004. 109:951–957.
41. Cloward TV, Walker JM, Farney RJ, Anderson JL. Left ventricular hypertrophy is a common echocardiographic abnormality in severe obstructive sleep apnea and reverses with nasal continuous positive airway pressure. Chest. 2003. 124:594–601.
42. Buchanan J, Mazumder PK, Hu P, et al. Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology. 2005. 146:5341–5349.
43. Mazumder PK, O'Neill BT, Roberts MW, et al. Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes. 2004. 53:2366–2374.
44. Coort SL, Hasselbaink DM, Koonen DP, et al. Enhanced sarcolemmal FAT/CD 36 content and triacylglycerol storage in cardiac myocytes from obese zucker rats. Diabetes. 2004. 53:1655–1663.
45. Semeniuk LM, Kryski AJ, Severson DL. Echocardiographic assessment of cardiac function in diabetic db/db and transgenic db/db-hGLUT4 mice. Am J Physiol Heart Circ Physiol. 2002. 283:H976–H982.
46. Boudina S, Sena S, Theobald H, et al. Mitochondrial energetics in the heart in obesity related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes. 2007. 56:2457–2466.
47. Unger RH. Lipotoxic disease. Annu Rev Med. 2002. 53:319–336.
48. Zhou YT, Grayburn P, Karim A, et al. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci U S A. 2000. 97:1784–1789.
49. Sidell RJ, Cole MA, Draper NJ, Desrois M, Buckingham RE, Clarke K. Thiazolidinedione treatment normalizes insulin resistance and ischemic injury in the Zucker fatty rat heart. Diabetes. 2002. 51:1110–1117.
50. Ruano M, Silvestre V, Castro R, et al. Morbid obesity, hypertensive disease and the rennin-angiotensin-aldosterone axis. Obes Surg. 2005. 15:670–676.
51. Hainault I, Nebout G, Turban S, Ardouin B, Ferre P, Quiqnard-Boulange A. Adipose tissue-specific increase in angiotensinogen expression and secretion in the obese (fa/fa) Zucker rat. Am J Physiol Endocrinol Metab. 2002. 282:E59–E66.
52. Davy KP, Hall JE. Obesity and hypertension: two epidemics or one? Am J Physiol Regul Integr Comp Physiol. 2004. 286:R803–R813.
53. Miner EC, Miller WL. A look between the cardiomyocytes: the extracellular matrix in heart failure. Mayo Clin Proc. 2006. 81:71–76.
54. Quilliot D, Alla F, Bohme P, et al. Myocardial collagen turnover in normotensive obese patients: relation to insulin resistance. Int J Obes (Lond). 2005. 29:1321–1328.
55. Carroll JF, Tyagi SC. Extracellular matrix remodeling in the heart of the homocysteinemic obese rabbit. Am J Hypertens. 2005. 18:692–698.
56. Halaas JL, Gajiwala KS, Maffei M, et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995. 269:543–546.
57. Madani S, De Girolamo S, Munoz DM, Li RK, Sweeney G. Direct effects of leptin on size and extracellular matrix components of human pediatric ventricular myocytes. Cardiovasc Res. 2006. 69:716–725.
58. Park S, Cho YR, Kim HJ, et al. Unraveling the temporal pattern of diet-induced insulin resistance in individual organs and cardiac dysfunction in C57BL/6 mice. Diabetes. 2005. 54:3530–3540.
59. Purdham D, Zou MX, Rajapurohitam V, Karmazyn M. Rat heart is a site of leptin production and action. Am J Physiol Heart Circ Physiol. 2004. 287:H2877–H2884.
60. Kumada M, Kihara S, Ouchi N, et al. Adiponectin specifically increased TIMP-1 through interleukin-10 expression in human macrophages. Circulation. 2004. 109:2046–2049.
61. Olivetti G, Abbi R, Quaini F, et al. Apoptosis in the failing human heart. N Engl J Med. 1997. 336:1131–1141.
62. Communal C, Sumandea M, de Tombe P, Narula J, Solaro RJ, Hajjar RJ. Functional consequences of caspase activation in cardiac myocytes. Proc Natl Acad Sci U S A. 2002. 99:6252–6256.
63. Cigola E, Kajstura J, Li B, Meggs LG, Anversa P. Angiotensin II activates programmed myocyte cell death in vitro. Exp Cell Res. 1997. 231:363–371.
64. Communal C, Singh K, Sawyer DB, Colucci WS. Opposing effects of beta 1- and beta2-adrenergic receptors on cardiac myocyte apoptosis: role of a pertussis-toxin sensitive G protein. Circulation. 1999. 100:2210–2212.
65. Relling DP, Esberg LB, Fang CX, et al. High-fat diet-induced juvenile obesity leads to cardiomyocyte dysfunction and upregulation of Foxo3a transcription factor independent of lipotoxicity and apoptosis. J Hypertens. 2006. 24:549–561.
66. Alpert MA, Lambert CR, Panayiotou H, et al. Relation of duration of morbid obesity to left ventricular mass, systolic function, and diastolic filling, and effect of weight loss. Am J Cardiol. 1995. 76:1194–1197.
67. Klein S, Fontana L, Young VL, et al. Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. N Engl J Med. 2004. 350:2549–2557.
68. Torgerson JS, Hauptman J, Boldrin MN, Sjostrom L. Xenical in the prevention of diabetes in obese subjects (XENDOS) study. Diabetes Care. 2004. 27:155–161.
69. Wadden TA, Berkowitz RI, Womble LG, et al. Randomised trial of lifestyle modification and pharmacotherapy for obesity. N Engl J Med. 2005. 353:2111–2120.
70. Curioni C, Andre C. Rimonabant for overweight or obesity. Cochrane Database Syst Rev. 2006. 4:CD006162.
71. Padwal RS, Majumdar SR. Drug treatments for obesity: oristat, sibutramine, and rimonabant. Lancet. 2007. 369:71–77.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download