Abstract
Swine-origin influenza A (H1N1) is caused by a new strain of the influenza virus. The disease has spread rapidly and was declared a pandemic in April, 2009. So far, however, there is a scarcity of information regarding the complications of swine influenza. A report of the disease in the winter of 2009 in the Southern Hemisphere found that the most common manifestations of influenza A virus infection are upper respiratory tract infection and pneumonia. Although there may be an association between fulminant myocarditis and Swine influenza, cardiovascular complications resulting from swine Influenza A infection are exceedingly rare. We report a case of acute constrictive pericarditis in a healthy subject infected by the swine-origin influenza A (H1N1) virus.
Recently, a flu pandemic occurred after a new influenza A (H1N1) virus appeared in humans in Mexico. By the end of the 2008-2009 influenza season, the virus had spread throughout the Northern hemisphere.1) Since April, 2009, the number of H1N1-infected patients has been rapidly increasing in South Korea. The most common manifestation of swine-origin influenza A is a respiratory tract infection. While the majority of people only experience mild symptoms, serious complications such as acute respiratory distress syndrome and pneumonia can occur. Recently, a single case of fulminant myocarditis was found in a child infected with swine influenza A.2) We report a case of acute transient constrictive pericarditis in a healthy adult infected with swine influenza A.
A 40-year-old man came to the emergency department complaining of a persistent fever and a sore throat for 8 days, with chest pain beginning 2 days prior to admission. The pain increased when inhaling deeply or assuming a left decubitus position. Eight days prior to admission, he had visited another hospital after 2 days of a fever and a sore throat. An H1N1 viral real-time RT-PCR test (by nasopharyngeal swab) confirmed the presence of H1N1 viral infection. He had been prescribed oseltamivir (Tamiflu™) for 5 days. During the first 4 days of taking oseltamivir, fever and sore throat improved. However, 2 days prior to coming to our emergency department, he had a high spiking fever and also felt an onset of severe chest pain, aggravated by deep breathing and assuming a left decubitus position.
The patient had no other relevant medical history. Upon general examination, he appeared acutely ill. Blood pressure was normal (110 mmHg systolic and 80 mmHg diastolic), heart rate was 90 beats per minute, and body temperature was 39.3℃. Upon physical examination, a regular heart beat, without a murmur or precordial friction rubbing, was heard and the lung sound was clear without rale.
A chest radiograph showed normal lung parenchyma and normal heart size. A 12-lead electrocardiogram showed concave ST segment elevation in the range of V2 to V5 pre-cordial leads (Fig. 1). Laboratory investigations upon admission revealed active inflammation with myocardial injury: white blood cell count 25,500/L (neutrophil 88.7%); C-reactive protein, 303 mg/L (0-8 mg/L); Creatine phosphokinase, 30 IU/L (44-245 IU/L); Creatine phosphokinase-MB, 1.77 ng/mL (0.0-5.0 ng/mL); troponin-T, 0.186 ng/mL (0.0-0.1 ng/mL); and normal chemistry profile and liver function tests. Blood culture was sterile.
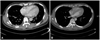
A transthoracic echocardiogram revealed a small pericardial effusion without evidence of hemodynamic compromise. There was no discernible regional wall motion abnormality, but abnormal septal wall motion and respiratory variation (>25%) in the mitral E velocity suggested constrictive physiology (Fig. 2). Chest computed tomography also revealed a small pericardial effusion with increased pericardial thickness (Fig. 3).
The patient was started on aspirin (100 mg qd), ibuprofen (200 mg tid) and empirical antibiotics. After 5 days of treatment, the patient showed clinical improvement and the elevated ST segment was normalized (Fig. 1). The patient had an intermittent spiking fever every three to four days after clinical improvement, so a low-to-medium dose of steroid (prednisolone 10 mg once daily) was added for consideration of transient constrictive pericarditis. After administration of prednisolone, the spiking fever was subsequently resolved and a follow-up echocardiogram and computed tomography showed a complete resolution of the pericardial effusion and constrictive physiology (Figs. 2 and 3). The patient was discharged with the same oral medication. No clinical evidence of recurrence was found during the 3 month follow-up period.
Influenza A virus has a trend towards antigen shift, leading to influenza pandemics generally occurring every 30 to 40 years; it has been nearly 40 years since the last one (1968-1969). When bird flu was discovered in Hong-Kong in 1997, the World Health Organization (WHO) warned of a possible human influenza pandemic.3) Since that time, intermittent mutations of influenza A virus were reported; however, none of these led to a human pandemic. In March, 2009, a novel strain of swine-origin influenza A (H1N1) caused human infection, but it was not confirmed that this novel influenza virus could transfer from person-to-person.
On April 15th and 17th, 2009, the Centers for Disease Control and Prevention confirmed the first two cases of human infection with pandemic influenza virus in the United States. On April 25th, 2009, the WHO declared a world-wide emergency caused by respiratory disease. Influenza virus spread rapidly from person-to-person and the WHO declared a global flu pandemic on June 11th, 2009. As of November 20th, 2009, H1N1 has infected 526,060 patients worldwide and killed 6,770.4) According to domestic South Korean statistics, H1N1 has infected 57,606 patients and killed 104 patients.5) Infection may rapidly increase during the winter because of low group-acquired immunity.
Common features of seasonal influenza A include fever, cough, sore throat, myalgia, pneumonia, asthma, and bronchitis. However, the range of complications from swine-origin influenza virus is not well known. According to reports of hospitalized patients with H1N1 influenza in the United States from April to June, 2009, the most common complication from influenza was pneumonia (40% of patients),6) followed by bacterial co-infection and acute respiratory distress syndrome. Four children in Texas with pandemic H1N1 influenza A had seizures, but all four recovered fully.7)
Although some case reports noted myocarditis with seasonal influenza8-10) and a single report of fulminant myocarditis associated with Swine influenza was discovered in a child February 10, 2010, constrictive pericarditis during swine Influenza A infection hads not been reported. The development of constrictive hemodynamics and its subsequent resolution with medical therapy was first described as transient constrictive pericarditis by Sagrista-Sauleda et al.11) in 1987. Constrictive pericarditis is diagnosed by specific findings in echocardiography12)13) and cardiac MRI.14) The pathophysiology of transient constrictive pericarditis is not clearly confirmed but may be due to transient thickness and loss of elasticity caused by inflammation, fibrin deposition, and edema.14) The definitive treatment for constrictive pericarditis is pericardiectomy,15) but according to Haley et al.16) transient constrictive pericarditis can be resolved in 3 months by conservative medical treatment, including NSAIDs or steroids. If constrictive pericarditis is indicated in a hemodynamically stable patient, medical treatment should be considered over pericardiectomy in the early phase.
Acute pericarditis as a complication of H1N1 influenza infection is a very rare event. However, the clinical course and response to medical treatment showed no difference from acute pericarditis due to any other cause. This experience can be a reference for diagnosis and treatment of pericarditis complicated by novel influenza infection.
Figures and Tables
Fig. 1
Electrocardiogram in acute pericarditis. Diffuse ST segment elevation (A) 8 days after hospitalization, normalized ST segment (B).
References
1. Webb SA, Pettila V, Seppelt I, et al. Critical care services and 2009 H1N1 influenza in Australia and New Zealand. N Engl J Med. 2009. 361:1925–1934.
2. Bratincsák A, El-Said HG, Bradley JS, Shayan K, Grossfeld PD, Cannavino CR. Fulminant myocarditis associated with pandemic H1N1 influenza A virus in children. J Am Coll Cardiol. 2010. 55:928–929.
3. Coker R, Mounier-Jack S. Pandemic influenza preparedness in the Asia-Pacific region. Lancet. 2006. 368:886–889.
4. Pandemic (H1N1) 2009-update 75. Accessed Nov 20, 2009. geneva: World health Organization;Available from: http://www.who.int/csr/don/2009_11_20a/en/index.html.
5. Pandemic (H1N1) 2009-update November, 21, 2009. Korea Center for Disease Control and Prevention. Accessed Nov 21, 2009. Available from: http://flu.cdc.go.kr/sub/h1n1OntogenyStatus.jsp.
6. Jain S, Kamimoto L, Bramley AM, et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N Engl J Med. 2009. 361:1935–1944.
7. Centers for Disease Control and Prevention (CDC). Neurologic complications associated with novel influenza A (H1N1) virus infection in children - Dallas, Texas, May 2009. MMWR Morb Mortal Wkly Rep. 2009. 58:773–778.
8. Kitaura Y, Deguchi H, Terasaki F, Ukimura A, Morita H, Tatsumi T. Influenza myocarditis: pathophysiology and developmental mechanism of myocarditis. Nippon Rinsho. 2003. 61:1945–1952.
9. Mamas MA, Nair S, Fraser D. Cardiac tamponade and heart failure as a presentation of influenza. Exp Clin Cardiol. 2007. 12:214–216.
10. Nolte KB, Alakija P, Oty G, et al. Influenza A virus infection complicated by fatal myocarditis. Am J Forensic Med Pathol. 2000. 21:375–379.
11. Sagrista-Sauleda J, Permanyer-Miralda G, Candell-Riera J, Angel J, Soler-Soler J. Transient cardiac constriction: an unrecognized pattern of evolution in effusive acute idiopathic pericarditis. Am J Cardiol. 1987. 59:961–966.
12. Ha CB, Huh JY, Shin YW, Shin YK. Doppler flow patterns of constrictive pericarditis. Korean Circ J. 1989. 19:47–54.
13. Oh JK, Hatle LK, Mulvagh SL, Tajik AJ. Transient constrictive pericarditis: diagnosis by two-dimensional Doppler echocardiography. Mayo Clin Proc. 1993. 68:1158–1164.
14. Napolitano G, Pressacco J, Paquet E. Imaging features of constrictive pericarditis: beyond pericardial thickening. Can Assoc Radiol J. 2009. 60:40–46.
15. Kim CW. Constrictive pericarditis and pericardiectomy. Korean Circ J. 1979. 9:71–81.
16. Haley JH, Tajik AJ, Danielson GK, Schaff HV, Mulvagh SL, Oh JK. Transient constrictive pericarditis: causes and natural history. J Am Coll Cardiol. 2004. 43:271–275.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download