Abstract
Patients with hemophilia generally have a reduced frequency of coronary artery disease compared to the general population. As advances in the management of hemophilia have increased their life expectancy, the prevalence of coronary artery disease also has increased. However, there are no standard treatment guidelines for coronary artery disease in patients with hemophilia, especially in the field of coronary intervention. We report the case of a patient with severe hemophilia A who presented with acute coronary syndrome and was successfully treated with percutaneous coronary intervention.
Acute coronary syndrome is rare in patients with hemophilia, potentially due to shorter lifespan or perhaps because of coagulation factor VIII abnormality.1) Hemophilia A directly protects against the development of coronary artery disease. 2)3) However, patients with hemophilia might not be protected against atherosclerosis, as demonstrated by clinical studies4) and autopsy reports on hemophiliacs with fatal myocardial infarction showing extensive atherosclerotic lesions, but only rarely fresh thrombi.5) Girolami et al.5) studied 36 cases of acute coronary syndrome in patients with hemophilia A. In most cases, the event occurred during or after the infusion of recombinant factor VIII concentrates, desmopressin (DDAVP), and prothrombin complex concentrates.
The treatment of choice in patients with acute coronary syndrome is primary coronary angioplasty with stent implantation, although invasive treatment carries the risk of hemorrhagic adverse events. We report a case of hemophilia A with acute coronary syndrome, not precipitated by anticoagulation therapy, who was successfully treated by percutaneous transluminal coronary angioplasty with stent implantation.
A 52-year-old male patient presented to the emergency department with intermittent chest pain at rest. The patient had no cardiovascular risk factors except for a 20 pack-year history of smoking. The patient had severe hemophilia A diagnosed at 10 years of age. The patient reported that he had received coagulation factor VIII on occasion because of bleeding into the knee joints. Physical examination revealed a body temperature of 36.6℃, blood pressure 110/70 mmHg, pulse rate 70/minute, and respiration rate 20/minute.
The electrocardiogram (ECG) showed T-wave inversions in precordial leads V1 to V6 and slight elevation of the ST segment in precordial leads V1 and V2. The MB fraction of creatine kinase was 2.1 ng/ mL (normal, <5.0 ng/mL) and troponin-I was 0.194 ng/mL (normal, <0.5 ng/mL). Factor VIII activity was less than 1% (normal, 60-140%) and factor VIII inhibitor antibodies were 0.26 (normal, <0.01). Echocardiography showed regional wall motion abnormalities at the anterior and septal wall of the apex and preserved left ventricular systolic function with a 51.8% ejection fraction.
Chest pain decreased during infusion of isosorbide dinitrate, and the patient was admitted to the coronary care unit. After admission, a coronary CT scan was performed and showed significant narrowing in the proximal left anterior descending artery (LAD). Angina recurred one day later, even with optimal medical treatment. Therefore, the patient was prepared for coronary angiography (CAG) and angioplasty.
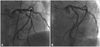
Following the advice of a hematologist, the patient received 2,500 IU of monoclonal coagulation factor VIII (Greenmono®, Baxter, Deerfield, IL, USA) prior to the procedure in addition to 1,500 IU of factor VIII at 12, 24, and 48 hours after the procedure. Catheterization was performed via the right femoral artery (Judkins technique). The patient received 70 IU/kg of unfractionated heparin and a loading dose of clopidogrel (300 mg). CAG revealed an 80% stenosis of the proximal segment and 40% in the mid segment of the LAD, some non-stenotic changes in the left circumflex, and a diminished right coronary artery (Fig. 1A). Balloon angioplasty was performed at the proximal LAD. However, the outcome was suboptimal and a cobalt-chromium coronary stent 3.5×18 mm (Vision®, Abbott Inc, Santa Clara, CA, USA) was implanted (Fig. 1B). To stop bleeding at the puncture site, we compressed the site manually for about 30 minutes. No local complications were observed and the patient did not complain of chest pain. The patient was discharged on aspirin 100 mg/d and clopidogrel 75 mg/d for 1 month, followed by long-term aspirin therapy 100 mg/d and regular coagulation factor VIII supplements.
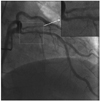
Ten months after the initial procedure, the patient was admitted to perform a follow-up CAG for evaluation of the patency of the implanted stent. The patient had taken aspirin, an angiotensin receptor blocker, nicorandil, and a calcium channel blocker, and also had received regular coagulation factor VIII supplements. He did not report any chest pain or any hemorrhagic complications. The echocardiography showed a normal ejection fraction (63.8%) without regional wall motion abnormalities. However, the CAG revealed 50% tubular concentric luminal narrowing of the proximal segment of the LAD stent (Fig. 2). The patient refused further evaluations and invasive treatment. Medical therapy has continued and the patient has remained asymptomatic.
The diagnosis of severe hemophilia in a patient with acute coronary syndrome should not delay invasive procedures and optimal medical therapy. The primary goal is to intervene before a full myocardial infarction. Anti-thrombotic treatment, particularly with percutaneous coronary intervention (PCI) and stent implantation, poses a therapeutic dilemma in patients with impaired coagulation. PCI requires the use of anticoagulants in patients with hemophilia, which can increase the risk of local complications.
Substituting the missing coagulation factor can reduce the bleeding risk during the procedure, even though it may increase the risk of acute thrombosis in a patient with unstable atherosclerotic plaques. According to the World Federation of Hemophilia recommendations, patients with hemophilia A that are going to undergo major surgery should be supplemented with factor VIII before the procedure to achieve the level of 80-100% of factor VIII activity. However, there is no similar protocol for coagulation supplementation prior to PCI.
Anticoagulation therapy is important to avoid thrombosis both from procedure-related complications and from further progression of coronary luminal narrowing; heparin is usually titrated to an optimal activated clotting time.6) Bivalirudin does not require monitoring and has a low rate of bleeding complications because it inhibits circulating and clot-bound thrombin directly, unlike heparin.7)8)
Antiplatelet therapies are as important as anticoagulation therapy for the prevention of acute thrombosis in an implanted stent;9) hemophilia is not associated with abnormalities of platelet number or platelet function. Current guidelines recommend dual antiplatelet therapy with aspirin and clopidogrel for at least 1 month for bare-metal stents and for at least 1 year for drug-eluting stents.10) However, antiplatelet therapy can increase the hemorrhagic tendency in these patients. Even with disturbed coagulation, antiplatelet therapy is important for hemophiliacs with PCI to prevent stent thrombosis. Bovenzi et al.11) reported a case of acute stent thrombosis in a patient with hemophilia B after coronary stent implantation who was not treated with aspirin or clopidogrel.
In our case, we used a bare-metal stent for early withdrawal of antiplatelet therapy. Aspirin as secondary prophylaxis against ischemic coronary events has been reported in patients with mild and moderate hemophilia.12) Here, long-term aspirin did not increase the incidence of hemorrhagic events, probably due to regular substitution of coagulation factor VIII. When long-term aspirin is needed in patients with severe hemophilia, Ferrario et al.13) suggested that coagulation factor VIII replacement should be prescribed also. Bare metal stents do not prevent neointimal hyperplasia. There are no reports on the use of drug-eluting stent implantation in patients with hemophilia; however, with concerns about bleeding diathesis, bare-metal stents are regarded as safe.
In conclusion, a PCI in a patient with severe hemophilia and acute coronary syndrome has acceptable complication risks. Antiplatelet therapy is important for preventing thrombus formation in the implanted stent even in patients with abnormal coagulation.
Figures and Tables
References
1. Plug I, van der Born JG, Peters M, et al. Mortality and causes of death in patients with hemophilia, 1992-2001: a prospective cohort study. J Thromb Haemost. 2006. 4:510–516.
2. Rosendaal FR, Briet E, Stibbe J, et al. Haemophilia protects against ischaemic heart disease: a study of risk factors. Br J Haematol. 1990. 75:525–530.
3. Srámek A, Kriek M, Rosendaal FR. Decreased mortality of ischaemic heart disease among carriers of haemophilia. Lancet. 2003. 362:351–354.
4. Srámek A, Reiber JH, Gerrits WB, Rosendaal FR. Decreased coagulability has no clinically relevant effect on atherogenesis: observations in individuals with a hereditary bleeding tendency. Circulation. 2001. 104:762–767.
5. Girolami A, Ruzzon E, Fabris F, Varvarikis C, Sartori R, Girolami B. Myocardial infarction and other arterial occlusions in hemophilia a patients. a cardiological evaluation of all 42 cases reported in the literature. Acta Haematol. 2006. 116:120–125.
6. Bond L, Bevan D. Myocardial infarction in a patient with hemophilia treated with DDAVP. N Engl J Med. 1988. 318:121.
7. Arora U, Dhir M, Cintron G, Strom JA. Successful multi-vessel percutaneous coronary intervention with bivalirudin in a patient with severe hemophilia A: a case report and review of literature. J Invasive Cardiol. 2004. 16:330–332.
8. Krolick MA. Successful percutaneous coronary intervention in a patient with severe haemophilia A using bivalirudin as the sole procedural anticoagulant. Haemophilia. 2005. 11:415–417.
9. Park DW, Park SW. Stent thrombosis in the era of the drug-eluting stent. Korean Circ J. 2005. 35:791–794.
10. Brener SJ, Steinhubl SR, Berger PB, Brennan DM, Topol EJ. Prolonged dual antiplatelet therapy after percutaneous coronary intervention reduces ischemic events without affecting the need for repeat revascularization: insights from the CREDO trial. J Invasive Cardiol. 2007. 19:287–290.
11. Bovenzi F, De Luca L, Signore N, Fusco F, de Luca I. Abciximab for the treatment of an acute thrombotic coronary occlusion during stent implantation in a patient with severe hemophilia B. Ital Heart J. 2003. 4:728–730.
12. MacKinlay N, Taper J, Renisson F, Rickard K. Cardiac surgery and catheterization in patients with haemophilia. Haemophilia. 2000. 6:84–88.
13. Ferrario C, Renders F, Cairoli A, Vuffray A, Spertini O, Angelillo-Scherrer A. Management of an acute coronary syndrome in a patient tvv with severe haemophilia A. Haemophilia. 2007. 13:763–765.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download