Abstract
Background and Objectives
The pathogenesis of hyponatremia (serum sodium <135 mEq/L) in Kawasaki disease (KD) remains unclear. We investigated the clinical significance of hyponatremia, and the role of interleukin (IL)-6 and IL-1β in the development of hyponatremia and syndrome of inappropriate antidiuretic hormone secretion (SIADH) in KD.
Subjects and Methods
Fifty KD patients were prospectively enrolled and analyzed for clinical and laboratory variables according to the presence of hyponatremia or SIADH.
Results
Thirteen KD patients (26%) had hyponatremia and 6 of these had SIADH. In patients with hyponatremia, the percentage of neutrophils (% neutrophils), C-reactive protein (CRP), and N-terminal pro-brain natriuretic peptide (NT-proBNP) were higher than in those without hyponatremia, while serum triiodothyronine (T3) and albumin were lower. Patients with hyponatremia had a higher incidence of intravenous immunoglobulin-resistance but this was not statistically significant. No differences existed between patients with and without SIADH with regard to clinical or laboratory variables and the incidence of IVIG-resistance. Serum sodium inversely correlated with % neutrophils, CRP, and NT-proBNP, and positively correlated with T3 and albumin. Serum IL-6 and IL-1β levels increased in KD patients and were higher in patients with hyponatremia. Plasma antidiuretic hormone increased in patients with SIADH, which tended to positively correlate with IL-6 and IL-1β levels.
Hyponatremia in Kawasaki disease (KD) was first reported in 1982,1) with occasional reports since then of KD associated hyponatremia and syndrome of inappropriate antidiuretic hormone secretion (SIADH).2-4) Multiple etiologies for hyponatremia in KD have been suggested1) and cerebral vasculitis has been thought to be related to SIADH.3) However, the precise pathogenesis of hyponatremia in KD has yet to be conclusively determined. Recently, it was revealed that many inflammatory cytokines such as interleukin (IL)-6 and IL-1β are elevated during the acute phase of KD,5-8) and a relationship between these cytokines and antidiuretic hormone (ADH) secretion has been suggested.9)10) It has therefore been speculated that these cytokines may be involved in the pathogenesis of hyponatremia due to SIADH in KD.
In this study, we investigated the clinical significance of hyponatremia in the acute phase of KD, and the role of IL-6 and IL-1β in the development of hyponatremia and SIADH associated with KD.
During the study period from November 2008 to July 2009, 54 children who met the diagnostic criteria for KD11) were prospectively enrolled and treated with intravenous immunoglobulin (IVIG), 2 g/kg and aspirin 80-100 mg/kg/day at the Department of Pediatrics, Ewha Womans University Mok-dong Hospital. Four of the 54 KD patients were excluded who had clinical signs of dehydration at the time of admission as they did not match the diagnostic criteria for SIADH. Thus, 50 KD patients (28 boys, 22 girls; mean age 29.0±23.7 months; range 3-89 months) were studied in this study. Thirty-five age- and sex-matched healthy children without fever served as the control group.
In KD patients, we measured complete blood cell count, serum sodium (Na) and osmolality, urine Na and osmolality, aspartate aminotransferase, alanine aminotransferase, albumin, C-reactive protein (CRP), N-terminal pro-brain natriuretic peptide (NT-proBNP), and triiodothyronine (T3) in the acute (2 to 9 days after the onset of disease; mean, 4.3±1.7 days) and subacute phase (3 to 9 days after IVIG infusion; mean, 5.9±2.6 days) of KD.
We divided the KD patients into hyponatremic and non-hyponatremic groups and divided hyponatremic KD patients into SIADH and non-SIADH groups. Between each group, we compared the clinical variables (age, gender, duration of fever at initial IVIG infusion, and the presence of diarrhea on admission), laboratory variables, the incidences of IVIG-resistance and coronary artery lesions (CALs).
Blood samples for determination of serum IL-6 and IL-1β levels were obtained from KD patients in the acute phase of the illness (before IVIG infusion) and from healthy controls, which were stored at -70℃ until measurement. Serum levels of IL-6 and IL-1β were measured by enzyme-linked immunosorbent assay (ELISA) using V-MAX 220 VAC ELISA reader (Molecular Devices, Sunnyvale, CA, USA), and the relationship between these cytokines and serum Na concentrations was investigated. Plasma ADH levels were measured in KD patients with hyponatremia by radioimmunoassay using Cobra II Gamma-counter (Hewlett-Packard, Meriden, CT, USA), and the relationship with cytokines was also investigated. This study was approved by the Institutional Review Board of Ewha Womans University Mokdong Hospital (#216-2) and written informed consents were obtained from the parents of each patient.
Hyponatremia was defined as a serum Na level <135 mEq/L and SIADH was defined as follows: 1) hyponatremia (serum Na <135 mEq/L), 2) hypotonicity (plasma osmolality <280 mOsm/kg), 3) inappropriately concentrated urine (>100 mOsm/kg), 4) an elevated urine Na concentration (>20 mEq/L), 5) nor-mal renal, adrenal, and thyroid function, and 6) absence of hy-povolemia or dehydration.12)
Duration of fever was defined as the time elapsed from the onset of disease to the start of initial IVIG infusion. As an exclusion criterion, dehydration was defined as weight loss >3% and/or the presence of clinical findings such as dry mucous membrane, decreased skin turgor, sunken anterior fontanel, or hemoconcentration (hematocrit >50%).13)
IVIG-resistance was defined when additional rescue therapies were required owing to persistent or recrudescent fever (≥38.0℃ or 100.4℉) at least 48 hours after the end of initial IVIG infusion. CALs were assessed by echocardiography and were defined by either an internal diameter of the coronary artery lumen >3 mm in a child <5 years or >4 mm in a child ≥5 years, the internal diameter of a segment being at least 1.5-times as large as that of an adjacent segment or when the lumen was clearly irregular.14)
All data were analyzed with Statistical Package for the Social Sciences (SPSS) for Windows software version 12.0 (SPSS Inc, Chicago, IL, USA). Descriptive statistics are presented as mean±standard deviation for continuous variables or as the number (percentage) of patients for categorical variables. Mann-Whitney test and Fisher's exact test were used for comparing variables between two independent groups. Wilcoxon signed rank test was used for comparing variables between pre- and post-IVIG infusion. Pearson's correlation and Spearman's correlation were used for correlations between each variable. A level of p<0.05 was considered as statistically significant.
Thirteen (26%) of 50 KD patients had hyponatremia before IVIG infusion. Six (46%) of the 13 KD patients with hyponatremia had SIADH. One of the 6 KD patients with SIADH had aseptic meningitis. Serum Na concentrations ranged from 125 to 134 mEq/L with a mean of 132.1±2.3 mEq/L before IVIG infusion, and increased to within the normal range after IVIG infusion (range, 135 to 138 mEq/L; mean, 136.4±1.2 mEq/L, p=0.001) in all patients except one male patient whose serum Na was 134 mEq/L (post-IVIG infusion). He exhibited a serum Na of 125 mEq/L (pre-IVIG infusion), which was the lowest concentration in the study (Fig. 1).
After IVIG infusion, hyponatremia newly occurred in 8 KD patients including 1 KD patient with SIADH. There were no KD patients who had developed hyponatremia before IVIG infusion. Serum Na concentrations ranged from 132 to 134 mEq/L (mean, 133.4±0.9 mEq/L). Four of the 8 KD patients had excessive administration of hypotonic maintenance fluid (135% to 150% of maintenance requirements proposed by Holliday and Segar15)) during the management of accompanying rotaviral gastroenteritis (n=2), urinary tract infection (n=1), and hepatitis (n=1), leading to dilutional hyponatremia.
We clarify that we were concerned about hyponatremia at the time of presentation, not that which developed after IVIG treatment. Therefore, all the reported results relate to hyponatremia at presentation.
Between KD patients with (n=13) and without hyponatremia (n=37), there was no difference in clinical variables including age, gender, and duration of fever at initial IVIG infusion, and the presence of diarrhea. Regarding laboratory vari-ables, percentage of neutrophils in white blood cells (% neutrophils), CRP, and NT-proBNP were higher, and serum T3 and albumin were lower in KD patients with hyponatremia than in those without hyponatremia.
The KD patients with hyponatremia had a higher incidence of IVIG-resistance but this was not statistically significant (p=0.065). There was no difference in the incidence of CALs between the two groups (Table 1).
Between hyponatremic patients with (n=6) and without SIADH (n=7), no differences existed in clinical variables (age, gender, duration of fever at initial IVIG infusion, and the presence of diarrhea), laboratory variables, and the incidence of IVIG-resistance (Table 2).
In 50 KD patients, serum Na inversely correlated with % neutrophils (r=-0.51, p=0.000) (not shown), CRP (r=-0.41, p=0.004) (Fig. 2A) and NT-proBNP (r=-0.47, p=0.001) (Fig. 2B). In contrast, serum Na had positive correlations with T3 (r=0.34, p=0.021) (Fig. 2C) and albumin (r=0.35, p=0.014) (not shown).
Of the 50 KD patients, there were 7 patients with IVIG-resistance: 5 patients were treated with additional IVIG (2 g/kg) and 2 patients with both IVIG and methylprednisolone pulse therapy. The KD patients with IVIG-resistance had a lower Na concentration (134.4±2.9 mEq/L vs. 136.3±2.8 mEq/L, p=0.065) and a higher incidence of hyponatremia {4/7 (57.1%) vs. 9/43 (20.9%), p=0.083} than those without IVIG-resistance, but with no statistical significance.
As for coronary complications, there were 4 patients with CALs, all of which were <5 mm in diameter. Between KD patients with and without CALs, there were no significant differences in serum Na levels (135.7±3.7 mEq/L vs. 136.1±2.9 mEq/L, p=1.000) and the incidence of hyponatremia {1/4 (25%) vs. 12/46 (26%), p=0.769}.
Serum IL-6 and IL-1β levels were measured in 42 KD patients and 35 healthy controls. There were no differences between the KD patients and the control group in regard to age (29.0±22.0 months vs. 20.6±18.4 months, p=0.062) and gender {male/female, 25/17 vs. 20/15, p=1.000}. However, serum levels of IL-6 and IL-1β were higher in KD patients than in healthy controls (IL-6, 89.6±74.2 pg/mL vs. 16.6±44.8 pg/mL, p=0.000; IL-1β, 45.0±71.0 pg/mL vs. 14.0±13.8 pg/mL, p=0.021) (Fig. 3A).
In the 42 KD patients, 11 patients with hyponatrema had higher levels of IL-6 and IL-1β than 31 patients without hyponatremia (IL-6, 184.3±77.8 pg/mL vs. 70.7±58.4 pg/mL, p=0.002; IL-1β, 104.6±143.7 pg/mL vs. 31.4±35.6 pg/mL, p=0.129) (Fig. 3B)
In the 11 KD patients with hyponatremia, serum IL-6 and IL-1β levels were higher in 5 KD patients with SIADH than in 6 patients without hyponatremia (IL-6, 210.0±88.6 pg/mL vs. 145.7±59.8 pg/mL, p=0.400; IL-1β, 150.6±182.2 pg/mL vs. 35.7±19.6 pg/mL, p=0.800), but with no statistical significance (Fig. 3C).
Plasma ADH levels (normal, <6.7 pg/mL) were measured in 7 KD patients with hyponatremia. Four patients with SIADH had higher plasma ADH levels compared with 3 patients without SIADH (17.0±8.2 pg/mL vs. 6.8±0.1 pg/mL, p=0.095). Plasma ADH concentrations positively correlated with serum levels of IL-6 (r=0.57, p=0.180) (Fig. 4A) and IL-1β (r=0.64, p=0.119) (Fig. 4B). However, this correlations were not statistically significant.
To detect the contributing factors to hyponatremia, five laboratory variables (% neutrophils, CRP, NT-proBNP, T3, and albumin) selected by the Mann-Whitney test plus two cytokines (IL-6 and IL-1β) were subjected to multivariate logistic regression analysis. No factors, however, were identified as independent contributors to hyponatremia in KD.
In the acute phase of the illness before IVIG infusion, hyponatremia occurred in 13 of 50 KD patients (26%) and SIADH was detected in 6 patients who comprised 46% of hyponatremic patients. The incidence of hyponatremia in this study was lower than those (43.3% to 54.5%) reported by others.1)4)13) As serum Na levels were ≥125 mEq/L, no symptomatic hyponatremia occurred. Without specific management, hyponatremia normalized in almost all cases. Hyponatremia and SIADH also developed in the subacute phase after IVIG infusion. However, such occurrences are usually due to extrinsic factors such as fluid therapy.
The clinical significance of hyponatremia in KD was investigated. The patients with hyponatremia had higher % neutrophils, CRP, and NT-proBNP levels, and lower T3 and albumin levels, indicating that hyponatremia occurs in patients exhibiting severe inflammation. Regarding T3 in KD, we have previously demonstrated that low T3 occurs in KD patients with higher CRP and NT-proBNP during the acute phase,16) suggesting that T3 also may be useful in monitoring the degree of inflammation together with CRP and NT-proBNP.17)18) Low albumin level has been recognized as a predictor of more severe KD with a higher risk of sequelae.19) The finding of hyponatremia being associated with severe inflammation is in close agreement with other studies.4)13) With regard to fever duration, there was no difference between patients with and without hyponatremia. This finding is inconsistent with other studies reporting a longer duration of fever in patients with hyponatremia.4)13) This difference could be attributed to hyponatremic patients with severe manifestations presenting to hospitals earlier than patients without mild hyponatremia. In fact, the time elapsed from the disease onset to initial treatment was approximately one day shorter in patients with hyponatremia than in those without. Considering the relationship between hyponatremia and IVIG-resistance, IVIG-resistance might occur more frequently in patients with hyponatremia, and hyponatremia might occur more commonly in patients with IVIG-resistance. Thus, hypon-atremia and IVIG-resistance may have a mutual relationship and hyponatremia may serve as a predictor of IVIG-resistance as described previously.20) In contrast, there was no correlation between hyponatremia and CALs in this study, which was in contrast to the results of other reports.4)13) This difference was probably due to the small number of patients who had CALs in our study. Contrary to hyponatremia, the presence of SIADH was not associated with severe inflammation or IVIG-resistance.
Several theories have been put forward as etiologies of hyponatremia: hyponatremic dehydration, ingestion of hypoosmolar fluid relative to excessive fluid loss,1) SIADH,1)3) and renal salt wasting.13)21) Recently, as it has been recognized that ADH secretion may be activated by IL-6 and IL-1β,9)10) involvement of these cytokines was focused on as a pathogenesis of hyponatremia due to SIADH. Mastorakos et al.9) have demonstrated increased ADH levels after IL-6 injection to cancer patients, indicative of stimulation of ADH secretion from magnocellular neurons by IL-6. Ohta and Ito10) have reported 4 cases of hyponatremia due to SIADH associated with inflammation. Hyponatremia occurred when the patients had fever and high CRP levels. Simultaneously, IL-6 and ADH levels were elevated with a significant correlation between them. They also demonstrated in animal studies that intravenous administration of IL-1β increases ADH, atrial natriuretic peptide, adrenocorticotropic hormone, and urinary Na excretion. As one of the main endocrine inflammatory cytokines IL-6 is involved in the de novo production of acute phase proteins by hepatocytes, causes fever in response to tissue injury, and plays a central role in endothelial damage in KD.5) Also, IL-1β acts as a mediator of endothelial damage in KD.22) Therefore, it is conceivable that these cytokines may be involved in the pathogenic mechanisms of hyponatremia and SIADH associated with KD.
We confirmed increased IL-6 and IL-1β levels during the acute phase of KD as reported in previous studies5-8) and that KD patients with hyponatremia have higher levels of these cytokines compared with those without hyponatremia. In patients with SIADH, these cytokines and ADH levels might be higher than in those without. In addition, there was a tendency of positive correlation between cytokines and ADH. These findings indicate that increased levels of serum IL-6 and IL-1β in acute KD may activate ADH secretion, leading to SIADH and hyponatremia.
With regard to the pathogenesis of hyponatremia in KD, several mechanisms may be suggested. KD is a systemic vasculitis and increased microvascular permeability is an initial step of the disease, causing hypoalbuminemia and noncardiogenic edema.23) Vascular leakage induces decreased intravascular volume and then activation of baroreceptors, leading to increased ADH secretion and hyponatremia.24) This is an appropriate pathophysiological response to restore extracellular fluid volume at the expense of hypoosmolarity. Hyponatremia also occurs as a result of ADH secretion that is inappropriate to both osmotic and fluid stimuli. Hyponatremia may result from increased natriuresis by IL-1β10) and natriuretic peptide activity,25)26) or from renal salt wasting due to either renal involvement13)27) or a reduction in renal Na absorption.21) It may also be caused by dilution of extracellular fluid due to impaired free water excretion, frequently occurring during fever and severe infections.28) The precise mechanisms for hyponatremia and SIADH in KD are not fully understood, but several factors could be active in the same patient.
The study has several limitations. First, as a prospective study, it was difficult to enroll a large patient population during a short study period. Because of the small number of subjects in each group, no statistical differences between groups existed with respect to incidence of CALs, cytokines and ADH levels, nor did ADH statistically correlate with cytokine levels. Secondly, because there were no KD patients who had CALs >5 mm in diameter, we were not able to investigate serum Na levels in patients with more severe coronary complications. Finally, although the results suggest that serum IL-6 and IL-1β play a key role in the pathogenesis of hyponatremia due to SIADH, these cytokines actually did not prove to be independent determinants of hyponatremia. This may have been partly due to the fact that hyponatremia secondary to cytokine-mediated ADH secretion occurred in only 46% of all cases.
Despite these limitations, this study indicates that hyponatremia in KD occurs in patients exhibiting severe inflammation. Hyponatremia may serve as a predictor of IVIG-resistance. As a possible pathogenesis model, we suggest that increased IL-6 and IL-1β levels during the acute phase of KD may activate ADH secretion, leading to SIADH and hyponatremia. Larger studies are needed to clarify the pathogenic role of IL-6 and IL-1β on hyponatremia and SIADH in KD patients.
Figures and Tables
Fig. 1
Changes in serum sodium (Na) level in 13 Kawasaki disease patients with hyponatremia (serum Na <135 mEq/L) before and after intravenous immunoglobulin (IVIG) infusion.

Fig. 2
Correlations between serum sodium (Na) and (A) C-reactive protein (CRP), (B) N-terminal pro-brain natriuretic peptide (NT-proBNP), and (C) triiodothyronine (T3) in 50 patients with acute Kawasaki disease.

Fig. 3
Comparison in serum levels of interleukin (IL)-6 and IL-1β between (A) Kawasaki disease (KD) patients and the control group (Control), (B) KD patients with (HypoNa) and without (Non-hypoNa) hyponatremia, and (C) hyponatremic KD patients with (SIADH) and without (Non-SIADH). SIADH: syndrome of inappropriate antidiuretic hormone secretion.

Fig. 4
Correlation of antidiuretic hormone (ADH) with serum levels of (A) interleukin (IL)-6 and (B) IL-1β in 7 Kawasaki disease patients with hyponatremia.

Table 1
Clinical and laboratory variables in Kawasaki disease patients with and without hyponatremia
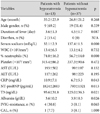
Table 2
Clinical and laboratory variables in hyponatremic Kawasaki disease patients with and without SIADH
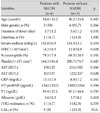
SIADH: syndrome of inappropriate antidiuretic hormone secretion, WBC: white blood cell, AST: aspartate aminotransferase, ALT: alanine aminotransferase, CRP: C-reactive protein, NT-proBNP: N-terminal pro-brain natriuretic peptide, T3: triiodothyronine, IVIG: intravenous immunoglobulin, CAL: coronary artery lesion, N/A: not applicable
References
1. Laxer RM, Petty RE. Hyponatremia in Kawasaki disease. Pediatrics. 1982. 70:655.
2. Lapointe N, Chad Z, Lacroix J, et al. Kawasaki disease: association with uveitis in seven patients. Pediatrics. 1982. 69:376–378.
3. Mine K, Takaya J, Hasui M, Ikemoto Y, Teraguchi M, Kobayashi Y. A case of Kawasaki disease associated with syndrome of inappropriate secretion of antidiuretic hormone. Acta Paediatr. 2004. 93:1547–1549.
4. Nakabayashi Y, Shimizu T. Hyponatremia in Kawasaki disease. Nippon Shoni Jinzobyo Gakkai Zasshi. 2002. 15:83–87.
5. Gupta M, Noel GJ, Schaefer M, Friedman D, Bussel J, Johann-Liang R. Cytokine modulation with immune gamma-globulin in peripheral blood of normal children and its implications in Kawasaki disease treatment. J Clin Immunol. 2001. 21:193–199.
6. Kim DS. Serum interleukin-6 in Kawasaki disease. Yonsei Med J. 1992. 33:183–188.
7. Maury CP, Salo E, Pelkonen P. Circulating interleukin-1beta in patients with Kawasaki disease. N Engl J Med. 1988. 319:1670–1671.
8. Suzuki H, Uemura S, Tone S, et al. Effects of immunoglobulin and gamma-interferon on the production of tumour necrosis factor-alpha and interleukin-1beta by peripheral blood monocytes in the acute phase of Kawasaki disease. Eur J Pediatr. 1996. 155:291–296.
9. Mastorakos G, Weber JS, Magiakou MA, Gunn H, Chrousos GP. Hypothalamic-pituitary-adrenal axis activation and stimulation of systemic vasopressin secretion by recombinant Interleukin-6 in humans: potential implications for the syndrome of inappropriate vasopressin secretion. J Clin Endocrinol Metab. 1994. 79:934–939.
10. Ohta M, Ito S. Hyponatremia and inflammation. Rinsho Byori. 1999. 47:408–416.
11. Dajani AS, Taubert KA, Gerber MA, et al. Diagnosis and therapy of Kawasaki disease in children. Circulation. 1993. 87:1776–1780.
12. Berry PL, Belsha CW. Hyponatremia. Pediatr Clin North Am. 1990. 37:351–363.
13. Watanabe T, Abe Y, Sato S, Uehara Y, Ikeno K, Abe T. Hyponatremia in Kawasaki disease. Pediatr Nephrol. 2006. 21:778–781.
14. Research committee on Kawasaki disease. Report of subcommittee on standardization of diagnostic criteria and reporting of coronary artery lesions in Kawasaki disease. 1984. Tokyo: Ministry of Health and Welfare.
15. Holliday MA, Segar WE. The maintenance need for water in parenteral fluid therapy. Pediatrics. 1957. 19:823–832.
16. Cho HK, Sohn JA, Kim HS, Sohn S. Low T3 syndrome in Kawasaki disease: relation to serum levels of tumor necrosis factor-alpha, interleukin-6 and NT-proBNP. Korean J Pediatr. 2009. 52:234–241.
17. Dahdah N, Siles A, Fournier A, et al. Natriuretic peptide as an adjunctive diagnostic test in the acute phase of Kawasaki disease. Pediatr Cardiol. 2009. 30:810–817.
18. Lee H, Kim H, Kim HS, Sohn S. NT-proBNP: a new diagnostic screening tool for Kawasaki disease. Korean J Pediatr. 2006. 49:539–544.
19. Harada K. Intravenous gamma-globulin treatment in Kawasaki disease. Acta Paediatr Jpn. 1991. 33:805–810.
20. Kobayashi T, Inoue Y, Takeuchi K, et al. Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease. Circulation. 2006. 113:2606–2612.
21. Eisenhut M. Changes in renal sodium transport during a systemic inflammatory response. Pediatr Nephrol. 2006. 21:1487–1488.
22. Smith PK, Goldwarer PN. Kawasaki disease in Adelaide: a review. J Paediatr Child Health. 1993. 29:126–131.
23. Terai M, Honda T, Yasukawa K, Higashi K, Hamada H, Kohno Y. Prognostic impact of vascular leakage in acute Kawasaki disease. Circulation. 2003. 108:325–330.
24. Koyanagi H, Nakamura Y, Yanagawa H. Lower level of serum potassium and higher level of C-reactive protein as an independent risk factor for giant aneurysms in Kawasaki disease. Acta Paediatr. 1998. 87:32–36.
25. Boomsma F, van den Meiracker AH. Plasma A- and B-type natriuretic peptides: physiology, methodology and clinical use. Cardiovasc Res. 2001. 51:442–449.
26. Fujiwara T, Fujiwara H, Takemura G, et al. Expression and distribution of atrial natriuretic polypeptide in the ventricles of children with myo-carditis and/or myocardial infarction secondary to Kawasaki disease: immunohistochemical study. Am Heart J. 1990. 120:612–618.
27. Ohta K, Seno A, Shintani N, et al. Increased levels of urinary interleukin-6 in Kawasaki disease. Eur J Pediatr. 1993. 152:647–649.
28. Duke T, Molyneux EM. Intravenous fluids for seriously ill children: time to reconsider. Lancet. 2003. 362:1320–1323.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download