Introduction
In the past, the epicardium was targeted for delivery of therapeutic agents, such as drugs, pacing leads, and devices in conjunction with an intended cardiac surgery to treat a variety of cardiovascular diseases via thoracotomy. The risk of potential complications related to thoracotomy made this approach less attractive for use as a routine procedure for non-cardiac surgery patients. In particular, the risk in patients who require multiple procedures can be significant, due to the extensive fibrosis as a result of a previous thoracotomy.
In recent years, invasive cardiac procedures via a closed pericardium have been proven to be safe and effective, particularly in the cardiac electrophysiology arena.1) Theoretically, a minimally invasive percutaneous approach that can provide the possibility of multiple procedures without causing significant fibrosis of the pericardium or other complications, may offer significant advantages for cardiac therapies performed via the epicardium. Other potential advantages of a pericardial approach are that it is possible to achieve a high concentration with longer duration of drugs due to the small confined space without any circulatory blood flow. Furthermore, it provides the views needed to guide delivery of an intended therapy, and minimize the risk of bleeding or strokes, because there is no need for anti-coagulation therapy.
The pericardial sac can be used as a diagnostic source for many cardiac and non-cardiac conditions, such as pericardial effusions from neoplastic or inflammatory processes. In some instances, epicardial biopsy guided by a pericardial scope would be useful for making a histological diagnosis.2)
The pericardium has much autonomic innervation and neuro-receptors that closely interact with cardiac contraction and coronary circulation. The mesothelial cells of the visceral pericardium have active metabolic activity, including cyclooxygenation and lipo-oxygenation. It is well known that prostanoids can alter the tone of the pericardial sympathetic neurons that influence myocardial contractility and coronary artery dilatation. There are many other potential physiologic roles that could be used for cardiac disease therapies.
However, as the currently practiced percutaneous cannulation technique for accessing a closed pericardium is still in the early stage of development, it has significant limitations and the technique needs to be tested for its safety and reproducibility. The important considerations of this review include the procedure for accessing a closed pericardial sac via the percutaneous approach, exploring the potential usage of this approach to treat cardiac diseases, and address the future development of new tools to meet the needs of the cardiovascular therapies.
Anatomy of Pericardial Sac
The pericardial sac consists of two layers with nerves, lymphatics, and blood vessels. The sac normally contains 20 to 40 mL of clear fluid that occupies the virtual space between the two layers, which are divided into the visceral and parietal pericardium. The visceral pericardium covers the entire epicardial surface, except for a small area on the posterior wall of the atria, and is composed of a single layer of mesothelial cells. The pericardial sac folds cover the major proximal vessels and the entire epicardial surface is accessible from the pericardial space, except for the atrial and ventricular septa which are not in direct contact with the pericardium.3)
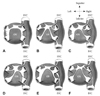
The pericardial sac has attachments to the posterior mediastinum formed by the oblique and transverse sinuses, which in turn form several recesses that fold over the great arteries and veins4-8) as shown in Fig. 1.9) There are several anatomical variations of these recesses, as shown in Fig. 2.10)
The layer not attached to the epicardium is the parietal pericardium. The parietal pericardium is much thicker and is composed predominantly of acellular fibrotic tissue that is rich in collagen. However, it has blood vessels and a dense innervation, including the autonomic and mechano-chemical receptors. The parietal pericardium is firmly attached to the anterior mediastinum and diaphragm through tendon-like fibrotic tissues. These attachments are essential to maintain normal cardiac position in relation to the surrounding structures, to restrict the volume of thin-walled cardiac chambers (right ventricle and right atrium), and also to serve as direct protection against injuries.11) The pericardium secrets several prostaglandin-like substances that may play an important role in the control of the autonomic and coronary vascular tones.12)
Pericardial Sac Cannulation
Clinical considerations
Percutaneous access of a closed pericardial sac has been successfully performed by the endocardial and epicardial approaches. The endocardial approach can be achieved by a puncture of the tip of the right atrial appendage with a small caliber needle, which enables access to the anterior region of the pericardial sac. Contrast can be injected to confirm the pericardial space. A guide-wire can then be placed and using an over-the-guidewire technique, a gradual dilatation of the puncture site can be performed to insert the desired diameter of sheath.13) Two epicardial approaches have been reported. One of which is the anterior parasternal approach to access the anterior region of the pericardial sac through the left intercostal spaces, which is less frequently practiced due higher complications observed. The most frequently used method is the sub-xyphoid approach reported by Sosa et al.1) This technique is the preferred approach by most investigators, and has been the basis for future technological development. There are several studies reporting new technologies that enable safer percutaneous access of the pericardial space, but no large-scale clinical data is available.14)
There are several contra-indications or conditions associated with higher risk complication risks from percutaneous cannulation of a closed pericardial sac.
These conditions are discussed below:
Previous cardiac surgery or pericardial surgery including pericardiectomy
Previous cardiac surgery usually results in significant pericardial fibrosis, and the pericardial space is virtually replaced by fibrotic adhesions. Percutaneous cannulation of the pericardial sac is very difficult, even in the case of a successful cannulation, the manipulation of instruments is extremely limited and difficult. The preferred approach in these patients is a hybrid method and it is discussed in the subsequent pages.
Congenital absence of the pericardium
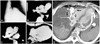
Congenital absence of the pericardium was recognized by Columbus15) and by Baille.16) These anomalies are the result of premature atrophy of the left duct of Cuvier, and premature reduction of blood supply to the left pleuropericardial membrane.17) The incidence is 1 in 14,000, and predominantly involves partial absence of the left pericardium.18) About one third of the cases occur with other congenital malformations, both cardiac and non-cardiac.19-21) Chest radiograph is key to diagnosis and has been the basis of the earliest clinical recognition of the congenital absence of the pericardium. The first diagnosis using chest radiograph was reported by Ellis et al.22) Chest radiograph findings can be confirmed with CT or MRI scans (Fig. 3A).23-25)
Pericardial varices
Pericardial varices are often observed in patients with proximal venous occlusive disease, particularly associated with superior vena cava syndrome.26) On rare occasions, portal hypertension can be associated with not only pericardial, but also mediastinal varices that can increase the risk of bleeding (Fig. 3B).
Pericardial cysts
Rare abnormalities in these areas are often diagnosed incidentally during routine chest radiographs that are mostly located in the apical regions, which can be confirmed by CT scan.27) The cysts can be large enough to interfere with cannulation.
Hiatal hernia
The presence of a hiatal hernia, especially of large size, can result in inadvertent perforation during cannulation attempts. Such perforation is often associated with mediastinal infections.
Ascites
Besides the bleeding risk associated with liver disease and pericardial varices, the trajectory of cannulation of the pericardial sac can unintentionally cause invasion of the abdominal cavity when penetrating the diaphragmatic aspect of the pericardial sac. The presence of a large ascites may result in post-procedural persistent drainage, which can be challenging to manage.
Complications associated with the pericardial cannulation
Percutaneous cannulation of a closed pericardium requires much experience in order to avoid complications. Several serious complications have been associated with this puncture technique, including hemothorax, cardiac perforation with hemopericardium, and abdominal viscus perforation. Rarely, complications can occur after successful cannulation due to mechanical trauma to the myocardium, including VT and VF, cardiac vein lacerations, and pericarditis.
Pre-procedure evaluation
Evaluation should include comprehensive clinical assessment and imaging studies to recognize any pre-existing contraindications, and to minimize complications. Chest radiograph, echocardiography, and cardiac MRI have proven to be useful for screening these patients.
Procedure environment
The procedure is usually performed in cardiac catheterization or electrophysiology laboratories. However, in view of the complexity of the patient's clinical condition and that of the procedure, a hybrid laboratory where cardiac surgery can be performed promptly would be the ideal setting.
Cannulation technique
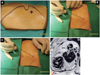
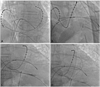
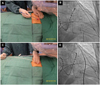
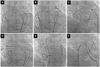
Intravenous antibiotics should be used routinely in these patients and given within an hour prior to the procedure. The components of the pericardial sac cannulation tray are shown in Fig. 4. The puncture site is identified based on the anatomical landmarks, as shown in Fig. 5A. The puncture needle approaches the site with a shallow angle in order to penetrate the skin and slide under the rib cage, and is re-oriented according to the targeted puncture site (Fig. 5B, C and D). The stylet is then removed, and a 10 mL syringe containing 1% lidocaine (3 to 5 mL) is attached to the proximal port of the needle. The needle is advanced gradually under fluoroscopy while infiltrating with lidocaine, which should be done after gentle aspiration to avoid injecting into any vessels. This maneuver is repeated until the parietal pericardium is reached. It is useful to confirm the needle orientation in three different views: anteroposterior, left anterior oblique (LAO), and right anterior oblique (RAO) views. The LAO view confirms the anterior versus posterior orientation (right and left cardiac chambers) (Fig. 6A) and the RAO view confirms the superior and inferior orientation (atria and ventricles) (Fig. 6B). Once the cardiac pulsation is felt with the needle tip, the lidocaine syringe is removed and a syringe that contains 2 to 3 mL of non-ionic contrast agent is attached. The puncture needle is carefully advanced toward the pericardial space, and often one can feel the "pop" as the needle penetrates the fibrotic parietal pericardial wall and then a small volume (<1 mL) of contrast is injected into the pericardial space to confirm successful puncture (Fig. 6C). A long soft "J" tip guide wire is then advanced far enough to silhouette the pericardial space (Fig. 6D).
The puncture needle is removed and an 8F dilator is advanced over the guide wire under fluoroscopic guidance (Fig. 7A and B) to achieve pre-dilatation prior to insertion of an 8.5F angled along the braided sheath (Swartz Braided SL1, St. Jude Medical, St. Paul, MN, USA) (Fig. 7C and D). The long sheath with the dilator is advanced or manipulated with the guide wire to reach the target area, followed by dilator removal. The angle of the sheath used should be less than 20° to avoid laceration of epicardial structures. The pericardial sheath is gently aspirated to obtain a yellowish clear pericardial fluid that confirms non-traumatic cannulation of the pericardial sac. Pericardial fluid can then be sampled for research purposes.
We performed a pericardiogram (Fig. 8) using 5 mL of a non-ionic contrast mixed with steroid (methylprednisolone 125 mg), and 5 mL of saline to guide epicardial mapping and minimize pericardial inflammation. Pericardiogram can be very useful for identifying important structures for future therapy. For example, the left atrial appendage can be easily visualized with fluoroscopy and can be a useful guide for epicardial ligation of the left atrial appendage to prevent thromboembolic events during atrial fibrillation. The pericardiogram can also be used to guide mapping catheter manipulation as shown in Fig. 9. The catheter or instruments can be manipulated manually or with an external magnetic field guided system, which may increase the accuracy and safety. During the procedure, blood pressure monitoring is important. If the systolic blood pressure drops more than 10 mmHg, the operator needs to rule out excessive pericardial fluid accumulation. This is especially important during open irrigated catheter ablation of cardiac arrhythmias. On occasion, we deliberately fill the pericardial space with 150 mL of saline to expand the pericardial space during epicardial ablation, in order to minimize collateral organ injuries (such as to the lungs, nerves, and esophagus).
Post-procedure care
After completion of the procedure, the sheath is gently aspirated to remove excess fluid, and to confirm the absence of bleeding. Generally, no major re-accumulation of pericardial fluid is observed following post-procedure draining of the pericardial fluid. Transthoracic echocardiogram is used to confirm complete drainage then the sheath is removed. We had a few patients who experienced persistent pericardial fluid drainage after the epicardial ablation procedure that required a "pig-tail" drainage catheter placement through the sheath, but usually drainage tapered quickly over the subsequent 24 to 48 hour period.
The catheter could be removed without any complications or re-accumulation. It is important to continue oral steroid and IV antibiotic therapy, and perform follow up echocardiogram studies in these patients.
Cardiac Therapies via a Closed Pericardium
Catheter ablation
There has been extensive data on open chest surgical ablation for ischemic ventricular tachycardia (VT) since the late 1980's, but subsequent development of implantable cardioverter defibrillator and percutaneous epicardial catheter ablation has drastically reduced the need for this technique in cardiac electrophysiology.
The first successful catheter based epicardial ablation of ischemic VT was reported by Sosa et al.30) Since then a larger number of cases of successful ablation has been reported.31) Subsequently, the safety and techniques for an efficient ablative energy delivery in the pericardial sac where cooling of the catheter tip is limited have been reported.32)33) Epicardial ablation has been shown to be essential in patients with VT related to structural heart disease, including ischemic heart disease, dilated cardiomyopathy, amyloidosis, and inheritable diseases such as arrhythmogenic right ventricular dysplasia.34) Recently, the pericardial approach has also been used to map and ablate non-cardiac tissue that plays an important role in triggering arrhythmia, such as the cardiac ganglionic plexi.35) Epicardial ablation via the pericardial approach has brought about higher success rate and safety in many cases that failed with endocardial ablation.
Hybrid approach
If the patient is unsuitable for percutaneous epicardial procedure due to previous cardiac surgery, catheter ablation can be performed via a pericardial window assisted by a surgical team in a hybrid laboratory setting of cardiac surgery and electrophysiology. There have been several case reports using this hybrid approach with good outcomes.36)
Drug delivery
There are many different cardiac drugs that have been delivered safely into the pericardial space, but the majority of these studies involving those drugs were conducted using the direct delivery methods via the open chest model in animals, or during cardiac surgery.37-41) The beneficial effects of nitric oxide and nitroprusside in ischemic animal model have been well studied.42)43) Zipes44) investigated voltage-sensitive chemicals for targeting depolarized cells in an ischemic model and calcium-avid cells that seek intracellular calcium. A myosin antibody loaded with anti-arrhythmic agents has been delivered via the pericardial space to demonstrate the efficacy. Avitall et al.45) used iontophoresis to enhance drug efficacy via the pericardial space.
Limited data is available on the closed pericardial sac model, especially in cardiac patients.46) Uchida et al.47) successfully delivered fibroblast growth factor into the pericardial space in an acute ischemic model to promote successful angiogenesis.
Laham et al.48) were also able to demonstrate successful angiogenesis effects of a bFGF delivery via a closed pericardial sac in ischemic heart disease patients. Closed pericardial sac drug therapy offers many advantages over the open chest model. Apart from safety and complication issues, a much higher local drug concentration can be achieved above the systemic tolerance range by intrapericardial delivery, and specific drugs that target the epicardium will be more effective.49)
Future Developments
Safety and technologies
The procedural environment needs to be defined and new tools need to be developed to improve safety and facilitate the procedure. A small caliber flexible non-traumatic scope that visualizes the entire procedure via a small subxyphoid incision would be ideal for assisting puncture of a closed pericardial sac. A large diameter scope is likely to increase pericardial inflammation, trauma, and scar tissue that can cause difficulties in future procedures. Tool design may vary according to the intended therapy, but a single device for multi-purpose use would be ideal for minimizing the cost and facilitating learning.
Future Procedures in Cardiac Therapies
Electrophysiology and pacing
Magnetic field guided robotic, epicardial mapping and ablation
The currently available or upcoming magneto-robotic systems for guiding ablation catheter can be applied in the pericardial procedure. The limited experience, including ours, has shown that the mobility of the catheter is much smoother and easier to manipulate in the pericardial space than that when accessed from the endocardium. An epicardial approach using a robotic system in suitable patients should be safer and more effective.
Epicardial left atrial appendage ligation for stroke prevention
Several techniques and technologies are available for left atrial appendage closure to prevent strokes via the endocardial or epicardial approaches. Leithauser and Park50) in a previous issue of this journal extensively reviewed the endovascular approaches and discussed their limitations. From the cardiac arrhythmia stand point, the epicardial ligation technique published by Friedman et al.51) is the most attractive, since it can also isolate the left atrial appendage electrically.
Pacemaker lead placement
Wireless pacemaker technologies have gained intense interest from many private industries, and this technology can offer a variety of advantages. Pericardial pacing with this technology would no longer be cardiac structure dependent, and could create pacing close to that of true physiologic condition, especially for re-synchronization purposes.
Biological therapies
The first successful catheter-based pericardial gene transfer mediated by adenoviral vectors was reported by March et al.52) Furthermore, direct epicardial gene delivery of skeletal muscle Na+ channel genes to improve Na+ channel function of diseased myocardial cells was shown to be effective in suppressing VT in a canine myocardial infarction model.53) That study used an open chest model to deliver genes with the paintbrush technique. The study demonstrated the feasibility of delivering other types of biological agents, such as cells and genetically engineered tissue for regenerative as well as anti-arrhythmic therapy purposes. Brugada syndrome would be an excellent model for gene transfer therapy via the pericardial space. The majority of these patients have structurally normal hearts, albeit with specific ion channel mutations that primarily affect the epicardium of right ventricular out flow tract. In addition, biological pacemaker therapy for the sick sinus syndrome could be an excellent study model for pericardial therapy, since the sinus node is primarily an epicardial structure and a closed pericardial sac that offer a better environment for biological pacemaker cells to anchor to the target tissue.54) However, the delivery of biological agents is the subject of ongoing investigation and is still far from clinical practice.
Conclusions
Percutaneous access to a closed pericardium is feasible and safe, and epicardial therapy has unique advantages in the variety of treatment modalities and lack of anatomical limitations. It should lead a shift in the treatment paradigm in the near future. However, percutaneous pericardial access requires extremely skilled technique and much operator experience. There is need for further refinement of the technique, design and development of dedicated equipment, and establishment of effective facilities.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download