Abstract
Background and Objectives
Right ventricle (RV) remodeling can determine tricuspid valve (TV) geometry and the severity of functional tricuspid regurgitation (TR).
Subjects and Methods
In 53 patients with various degrees of functional TR and in sinus rhythm, RV and TV geometries were analyzed using real-time 3-dimensional echocardiography, including tenting angles of 3 leaflets, septal-lateral and antero-posterior tricuspid annulus diameters and inlet RV dimensions, mid-RV septal-lateral dimension, and the distance between annulus and apex. A mid-systole frame when the TV tenting is smallest was selected for the analysis. RV end-diastolic and end-systolic volumes were measured. The severity of functional TR was determined by distal jet area.
Results
TR distal jet area was mainly determined by septal-lateral annulus diameter (p<0.001) RV inlet dimension (p=0.015), RV end-systolic volume (p=0.010), septal (p=0.019), and anterior leaflet tenting angles (p=0.045) by multiple stepwise linear regression analysis. Leaflet tenting angles were mainly determined by septal-lateral RV inlet dimension. Septal-lateral annulus diameter was determined by septal-lateral RV inlet dimension (p<0.001) and mid RV dimension (p=0.033), whereas antero-posterior annulus diameter was determined by antero-posterior RV inlet dimension (p<0.001).
Functional tricuspid regurgitation (TR) is caused by geometric changes of the tricuspid valve (TV) apparatus, including annulus dilation and leaflet tethering.1-5) These geometric deformations are linked with morphologic changes of the right ventricle (RV). However, the relationship between geometric changes of the RV and TV apparatus are unclear. Geometric components of RV remodeling that determine the severity of functional TR also remains to be clarified. Functional TR is usually treated with surgical tricuspid annuloplasty, which effectively reduces annulus size.6-8) Significant residual TR, however, are often found even after tricuspid annuloplasy,9)10) which can require additional surgical procedures. Understanding the RV geometric factors that determine the TV morphologic changes and the severity of TR may help solve this clinical problem. RV cannot be easily assessed geometrically compared to left ventricle (LV), making it difficult to analyze RV geometry using 2-dimensional echocardiography. Real-time 3-dimensional echocardiography (RT3DE) is a useful imaging tool for analyzing geometry of TV and RV.2)3)11) Therefore, we used RT3DE to evaluate the geometric factors of RV that determine morphologic changes in the TV apparatus and the severity of functional TR.
Two-dimensional echocardiography and RT3DE were performed in 53 patients (mean age: 54±17 years, 30 females) with various degrees of functional TR and in sinus rhythm. Associated cardiopulmonary diseases in these patients are described in Table 1. The distal jet area of TR on the 2-dimensional color Doppler image was used for quantification of TR severity.12)
RT3DE was performed to obtain a real-time 3-dimensional image of the TV from the low parasternal or apical view using SONOS 7500 or i33 instruments with a 2-4 MHz 3000-element xMATRIX transthoracic transducer (Philips, Andover, MA, USA). Images were obtained after the gain, compression controls, and time gain compensation settings were optimized to ensure image quality. Full-volume datasets were acquired in the wide-angled acquisition (93×80°) mode, in which four wedge-shaped subvolumes (93×20° each) were obtained from four different cardiac cycles during suspended respiration. Acquisition was triggered to the R wave of every other cardiac cycle to allow time for storage of each subvolume, resulting in a total acquisition time of seven heartbeats. Care was taken to include the TV annulus, valve leaflets, and entire RV in the full-volume image sets. The RT3DE images were digitally stored on a hard disk in the echocardiography instrument and transferred to a personal computer for offline analysis.
Using dedicated software (Tomtec, Munich, Germany), three longitudinal planes that perpendicularly crossed the middle of each leaflet were generated using guidance on the short axis image of the TV (Fig. 1A). On each of the three longitudinal planes, the leaflet tenting angles between the tricuspid annulus line and the three leaflets (anterior: Aα, posterior: Pα, septal: Sα) were measured on a mid-systole frame. Septal-lateral and antero-posterior longitudinal planes, which were mutually perpendicular, were created to measure septal-lateral and antero-posterior tricuspid annulus diameters (Fig. 1B). RV inlet dimensions were also measured 1 cm away from, and parallel to, the annulus lines, on both septal-lateral and antero-posterior planes. The septal-lateral mid RV dimension was measured at the mid portion between the TV annulus and RV apex. The antero-posterior mid RV dimension could not be measured because the RV flow tract is located on the anterior side. The distance between TV annulus and RV apex were measured on the septal-lateral and antero-posterior longitudinal planes. When these two values are not equal, the larger one was chosen. All measurement points marked on RT3DE still frames were verified by overlap with moving images. RV end-systolic and end-diastolic volumes were measured using 4D RV analysis (Tomtec, Munich, Germany) (Fig. 2), and RV ejection fraction was calculated.
Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS Inc, Chicago, IL, USA). Data are expressed as mean±SD. Multivariate analysis based on stepwise multiple linear regression analysis was implemented to discover independent determinants of the TR distal jet area or geometric factors of TV apparatus. A p <0.05 was considered significant.
The TR distal jet area significantly correlated with tenting angles of 3 leaflets, septal-lateral and antero-posterior annulus diameters and RV inlet dimensions, septal-lateral mid RV dimension, RV end-systolic volume, and RV ejection fraction (Table 2, Fig. 3). By multiple stepwise linear regression analysis, the septal-lateral annulus diameter (p<0.001), septal-lateral RV inlet dimension (p=0.015), RV end-systolic volume (p=0.010), Sα (p=0.019), and Aα (p=0.045) were independent determinants of TR distal jet area.
Among RV geometric factors, septal-lateral RV inlet dimension was the only determinant of Aα (Table 3). Septal-lateral RV inlet dimension was also the only determinant of Pα (Table 4). Sα was determined by septal-lateral RV inlet dimension and distance between TV annulus and RV apex (Table 5, Fig. 4). Septal-lateral annulus diameter was determined by septal-lateral RV inlet dimension and mid RV dimension (Table 6), whereas antero-posterior annulus diameter was determined by antero-posterior RV inlet dimension (Table 7, Fig. 5).
Patients with significant TR have poor prognosis.13)14) Significant TR alters the geometry of RV and LV, causing impaired left atrial contribution to left ventricular filling.15) Such RV remodeling seems to aggravate the severity of functional TR. We have demonstrated that functional TR severity is closely related with RV remodeling parameters such as RV inlet and mid RV dimension, distance between TV annulus and RV apex, and RV systolic volume and ejection fraction, as well as geometry of TV apparatus such as leaflet tenting and TV annulus diameters. Furthermore, septal-lateral annulus diameter, septal-lateral RV inlet dimension, RV end-systolic volume and Sα appeared to be major determinants of functional TR severity. These results suggest that RV and TV annular dilation towards RV free wall side and septal leaflet tethering are major geometric components that aggravate the severity of functional TR, consistent with previous reports analyzing morphologic changes of TV in patients with functional TR.2)3) The surgical strategy for functional TR, therefore, should be focused on reducing the septal-lateral dilation of TV annulus and RV inlet dimension, and on the relieving septal leaflet tethering. Ventricular enlargement due to RV volume overload by significant TR results in disproportionate dilation along the free wall to the septum minor axis and a more spherical RV shape.16) Such a dilation along the septal-lateral direction aggravated functional TR in return, producing a vicious cycle of increasing functional TR. RV remodeling and dysfunction may be reversed by surgical correction of TR.17)
The septal-lateral inlet RV dimension is the major determinant of tenting of all 3 leaflets, suggesting that leaflet tethering is more dependent on the septal-lateral RV dilation than antero-posterior RV dilation. Anatomic distribution of papillary muscles and subvalvular structure may be responsible for this phenomenon. Surgical or medical management to reverse and/or compensate septal-lateral RV dilation is optimal for reducing tethering of all 3 leaflets.
Septal-lateral and antero-posterior annulus dilations are dependent on the RV inlet dilations of the corresponding directions; septal-lateral annulus dimension was determined by septal-lateral RV inlet dimension and mid RV dimension, whereas antero-posterior annulus diameter was determined by antero-posterior RV inlet dimension. These results verify that TV annulus dilations are, at least in part, caused by RV dilations. Further study will be necessary to see if reversal of RV remodeling may reverse annulus dilation as well as leaflet tethering.
Although the surgical strategy of reducing free-wall dilation of RV for the treatment of functional TR has not been tested, it may improve surgical therapy with or without TV annuloplasty for patients with severe functional TR. Similar experimental surgical techniques for functional mitral regurgitation caused by left ventricular dilation may also be applied to patients with functional TR.18-20)
The geometric changes of RV associated with functional TR do not clarify causality. Functional TR may cause RV dilation and geometric remodeling, and vice versa. We describe the relationships between RV remodeling and morphology of the TV apparatus or severity of functional TR as part of the continuous remodeling process in a vicious cycle. We may not extrapolate the results to patients in atrial fibrillation because we only included patients in normal sinus rhythm. Full-volume images required for analyzing RV geometry could not be acquired in patients with atrial fibrillation with current RT3DE technology, but would be possible when a good full-volume image can be acquired in a single cardiac cycle.
In conclusion, we found using RT3DE that functional TR severity is determined by septal-lateral annulus and RV dilation, and tenting of septal and anterior leaflets. TV leaflet tenting is mainly determined by septal-lateral RV inlet dilation, and tricuspid annulus dilation is closely linked with inlet RV dilation of the corresponding directions.
Figures and Tables
Fig. 1
Geometric measurements using real-time 3-dimensional echocardiography. A: three longitudinal planes that perpendicularly cross the middle of each leaflet are generated by guidance on the short axis image of the tricuspid valve (upper panel). On each of these three longitudinal planes, the leaflet tenting angle between the tricuspid annulus line and the leaflet is measured on a mid-systole frame (lower panel). B: mutually perpendicular septal-lateral and antero-posterior longitudinal planes are created to measure septal-lateral and antero-posterior tricuspid annulus diameters, inlet right ventricular (RV) dimensions and mid RV septal-lateral dimension.
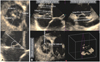
Fig. 2
Right ventricular (RV) volume measurement using real-time 3-dimensional echocardiography. RV end-systolic and end-diastolic volumes, and ejection fraction were measured using 4-dimensional RV analysis program.

Fig. 3
Correlations between distal jet area of tricuspid regurgitation (TR) and geometric variables of tricuspid valve and right ventricle (RV).

Fig. 4
Correlations between septal-lateral right ventricular (RV) inlet dimension and tenting angles of the 3 leaflets.

Fig. 5
Correlations between annulus diameters and right ventricular (RV) inlet dimensions of the corresponding directions.

Acknowledgments
This study was supported by a research grant of the Korean Society of Circulation (2007-7).
References
1. Sagie A, Schwammenthal E, Padial LR, Vazquez de Prada JA, Weyman AE, Levine RA. Determinants of functional tricuspid regurgitation in incomplete tricuspid valve closure: Doppler color flow study of 109 patients. J Am Coll Cardiol. 1994. 24:446–453.
2. Ton-Nu TT, Levine RA, Handschumacher MD, et al. Geometric determinants of functional tricuspid regurgitation: insights from 3-dimensional echocardiography. Circulation. 2006. 114:143–149.
3. Park YH, Song JM, Lee EY, Kim YJ, Kang DH, Song JK. Geometric and hemodynamic determinants of functional tricuspid regurgitation: a real-time three-dimensional echocardiography study. Int J Cardiol. 2008. 124:160–165.
4. Fukuda S, Saracino G, Matsumura Y, et al. Three-dimensional geometry of the tricuspid annulus in healthy subjects and in patients with functional tricuspid regurgitation: a real-time, 3-dimensional echocardiographic study. Circulation. 2006. 114:1 Suppl. I492–I498.
5. Sukmawan R, Watanabe N, Ogasawara Y, et al. Geometric changes of tricuspid valve tenting in tricuspid regurgitation secondary to pulmonary hypertension quantified by novel system with transthoracic real-time 3-dimensional echocardiography. J Am Soc Echocardiogr. 2007. 20:470–476.
6. Kanter KR, Doelling NR, Fyfe DA, Sharma S, Tam VK. De Vega tricuspid annuloplasty for tricuspid regurgitation in children. Ann Thorac Surg. 2001. 72:1344–1348.
7. Bonow RO, Carabello BA, Chatterjee K, et al. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2008. 118:e523–e661.
8. Kwon DA, Shin DH, Jung JW, et al. Echocardiographic parameters for predicting the outcome of patients undergoing surgery for severe tricuspid regurgitation. Korean Circ J. 2005. 35:916–920.
9. Fukuda S, Song JM, Gillinov AM, et al. Tricuspid valve tethering predicts residual tricuspid regurgitation after tricuspid annuloplasty. Circulation. 2005. 111:975–979.
10. Fukuda S, Gillinov AM, McCarthy PM, et al. Determinants of recurrent or residual functional tricuspid regurgitation after tricuspid annuloplasty. Circulation. 2006. 114:1 Suppl. I582–I587.
11. Tamborini G, Brusoni D, Torres Molina JE, et al. Feasibility of a new generation three-dimensional echocardiography for right ventricular volumetric and functional measurements. Am J Cardiol. 2008. 102:499–505.
12. Zoghbi WA, Enriquez-Sarano M, Foster E, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003. 16:777–802.
13. Koelling TM, Aaronson KD, Cody RJ, Bach DS, Armstrong WF. Prognostic significance of mitral regurgitation and tricuspid regurgitation in patients with left ventricular systolic dysfunction. Am Heart J. 2002. 144:524–529.
14. Nath J, Foster E, Heidenreich PA. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol. 2004. 43:405–409.
15. Louie EK, Bieniarz T, Moore AM, Levitsky S. Reduced atrial contribution to left ventricular filling in patients with severe tricuspid regurgitation after tricuspid valvulectomy: a Doppler echocardiographic study. J Am Coll Cardiol. 1990. 16:1617–1624.
16. Reynertson SI, Kundur R, Mullen GM, Costanzo MR, McKiernan TL, Louie EK. Asymmetry of right ventricular enlargement in response to tricuspid regurgitation. Circulation. 1999. 100:465–467.
17. Mukherjee D, Nader S, Olano A, Garcia MJ, Griffin BP. Improvement in right ventricular systolic function after surgical correction of isolated tricuspid regurgitation. J Am Soc Echocardiogr. 2000. 13:650–654.
18. Liel-Cohen N, Guerrero JL, Otsuji Y, et al. Design of a new surgical approach for ventricular remodeling to relieve ischemic mitral regurgitation: insights from 3-dimensional echocardiography. Circulation. 2000. 101:2756–2763.
19. Hung J, Guerrero JL, Handschumacher MD, Supple G, Sullivan S, Levine RA. Reverse ventricular remodeling reduces ischemic mitral regurgitation: echo-guided device application in the beating heart. Circulation. 2002. 106:2594–2600.
20. Inoue M, McCarthy PM, Popovic ZB, et al. The Coapsys device to treat functional mitral regurgitation: in vivo long-term canine study. J Thorac Cardiovasc Surg. 2004. 127:1068–1076. discussion 1076-7.




 PDF
PDF ePub
ePub Citation
Citation Print
Print








 XML Download
XML Download