Abstract
Most cases of cardiac metastasis from renal cell carcinoma (RCC) involve the vena cava or right atrium. Left ventricular metastases from RCC without involving the vena cava or right atrium are extremely rare. Herein we report a case of RCC with left ventricular metastasis causing left ventricular outflow obstruction (LVOT).
Secondary cardiac tumors are 20 to 40 times more frequent than primary cardiac malignancies.1) The most common secondary tumors of the heart originate from leukemia, melanoma, lung cancer, breast cancer, and lymphoma.2) Cardiac involvement may arise from hematogenous metastases, direct invasion from the mediastinum, or tumor growth into the vena cava and extension into the right atrium (RA). However, cardiac metastases from renal cell carcinoma (RCC) are rare, and in the absence of either direct vena caval or right cardiac extension, involvement of the left heart is extremely rare, with only a few known reports in the medical literature.3)
A 77-year-old man, who was diagnosed with RCC with peritoneal seeding 2 months previously (Fig. 1A and B), was admitted for severe dyspnea. He had been treated with sunitinib malate (Sutent, Pfizer, Korea) for only 3 days because of severe general weakness and poor oral intake. At the time of admission, his vital signs were unstable; his blood pressure was 78/46 mmHg, heart rate 136 beats per minute, and body temperature 36.7℃. Laboratory tests showed a normal leukocyte count (white blood cell, 7,450/µL), and pO2 of 72 mmHg and pCO2 of 33 mmHg on arterial blood gas analysis.
A 12-lead electrocardiogram revealed sinus tachycardia, and chest radiography findings included right hilar enlargement and reticular opacities in both lungs, in addition to cardiomegaly (Fig. 1C). Chest computed tomography (CT) revealed a large left ventricular mass involving the lateral wall; however, evidence of right ventricular or inferior venal caval involvement was not found (Fig. 1D). Multiple pulmonary nodules (Fig. 1E) and right hilar lymphadenopathy (Fig. 1F) were seen, suggesting pulmonary metastases.
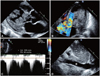
Two-dimensional transthoracic echocardiography showed a very large, lobulated, oscillating mass attached to the lateral wall and occupying the cavity of the left ventricle (LV) (Fig. 2A). This mass caused left ventricular outflow tract (LVOT) obstruction. Doppler imaging showed a turbulent color flow jet across the LVOT. The pressure gradient between the aorta and LV was 46 mmHg (Fig. 2B and C). There was no evidence of metastasis to the inferior vena cava or RA (Fig. 2D). The patient refused surgical treatment. On the following day, his blood pressure dropped to 56/35 mmHg, and he died from cardiogenic shock.
Cardiac metastases from RCC, are extremely rare, and usually occur by either of two mechanisms. The first employs a venous hematogenous pathway through the renal vein to the right heart. In patients with isolated disease and delayed disease progression to the right heart, with no involvement of the inferior vena cava, microdissemination through a venous hematogenous pathway remains the most probable mode of metastasis.4-6) The second pattern is through the lymphatic vessels of the thorax, involving the carinal lymph nodes that collect the drainage from the posterior wall of the heart, and then, through reversed lymphatic flow caused by metastasis to the nodes, metastatic RCC can spread to the pericardium and the left myocardium. Lymphatic drainage from the anterior and inferior walls of the heart passes through the parasternal and diaphragmatic lymph vessels. This hypothetical second pattern of RCC spread to the heart is supported by the observations that disseminated disease progression generally involves the supradiaphragmatic lymph nodes, lung, and pericardium, and the side of the heart more frequently involved is the left.6-8) The second pattern of spread is more compatible with our patient, because there was no involvement of the vena cava or RA, and at the time of diagnosis, a left ventricular mass causing LVOT obstruction was identified. Other possible causes of LVOT obstruction include metastasis of unknown origin, myxoma, rhabdomyoma, and rhabomyosarcoma. Although a biopsy of the left ventricular mass was not performed because of patient refusal, a secondary metastatic cardiac tumor of RCC is suspected, considering that there were multiple metastases in both lungs and the peritoneum.
About one-third of patients diagnosed with RCC develop metastatic disease, and metastatic RCC can be diagnosed as a solitary metastatic lesion or as widespread systemic disease. A solitary metastatic lesion eventually develops into a systemic pattern of recurrent disease.9) Although there have been advances in chemotherapy, the median survival time of patients with metastatic RCC is 6 to 12 months, and the 5-year survival rate is only 9%.10)
Metastatic RCC is highly resistant to chemotherapy, hormones, and radiotherapy, and the treatment of metastatic RCC remains ineffective. In the palliation of isolated, metastatic disease, surgical resection can play an important role. Currently, combination therapy that includes surgery and chemotherapy is the best chance of palliation and cure. Cardiac metastases from RCC in the absence of vena caval extension often grow slowly, tending to present many years after curative treatment. Every attempt should be made to resect these slow-growing metastatic lesions when they involve the heart. Coronary occlusion or compression from tumor masses can lead to myocardial infarction, eventual heart failure, and death. In our case, the patient refused surgical resection and had severe general weakness and cachexia; therefore the aggressive surgical removal of the left ventricular mass was impossible.
It is clear that although rare, renal cell cancer has the potential to metastasize to both the left and right ventricles, and cardiac metastasis should be considered in the clinical setting of renal cell cancer when the patient complains of sudden dyspnea of unknown origin and presents with sudden heart failure caused by LVOT obstruction.
Figures and Tables
Fig. 1
Renal cell carcinoma (RCC) with left ventricular and pulmonary metastases, but without right ventricular metastasis. A: abdominal computed tomography (CT) shows a very large RCC in the left kidney (white arrow) with thickening of the renal fascia. B: pathology obtained from the renal biopsy shows a typical clear cell renal cell carcinoma (H&E stain, ×200). C: plain chest radiograph shows cardiomegaly, right hilar enlargement, and reticular opacities in both lungs. D: chest CT shows a heterogenous mass lesion (white arrow) involving the entire left ventricle without right ventricular metastasis. E: multiple pulmonary nodules consistent with pulmonary metastases. Note underlying lung emphysema. F: a large metastatic lymph node in the right hilar area (white arrow).
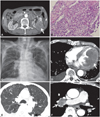
Fig. 2
Two-dimensional (2D) echocardiography of metastasis to the left ventricle (LV) from renal cell carcinoma, with no metastasis in the inferior vena cava (IVC). (A) 2D echocardiogram shows a very large, lobulated, oscillating mass attached to the lateral wall of the LV and occupying the cavity of the LV (B), and (C) color flow Doppler showing turbulent jet and continuous-wave Doppler velocity through the left ventricular outflow tract (LVOT). (D) No evidence of mass in IVC (*).
References
1. Silvestri F, Bussani R, Pavletic N, Mannone T. Metastases of the heart and pericardium. G Ital Cardiol. 1997. 27:1252–1255.
2. Neragi-Miandoab S, Kim J, Vlahakes GJ. Malignant tumors of the heart: a review of tumour type, diagnosis and therapy. Clin Oncol. 2007. 19:748–756.
3. Atik FA, Navia JL, Krishnamurthi V, et al. Solitary massive right ventricular metastasis of renal cell carcinoma without inferior vena cava or right atrium involvement. J Card Surg. 2006. 21:304–306.
4. Hunsaker RP, Stone JR. Images in clinical medicine: renal cell carcinoma extending into the vena cava and right side of the heart. N Engl J Med. 2001. 345:1676.
5. Kwon MJ, Kim DS, Kim AR, et al. A case of multiple metastatic renal cell carcinoma in an adult patient presenting with ventricular tachycardia. Korean Circ J. 2005. 35:341–344.
6. Zustovich F, Gottardo F, De Zorzi L, et al. Cardiac metastasis from renal cell carcinoma without inferior vena involvement: a review of the literature based on a case report: two different patterns of spread? Int J Clin Oncol. 2008. 13:271–274.
7. Eckman PM, Westensee JC, Missov E, Liao KK, Truskinovsky AM, Panetta C. Renal cell carcinoma in the left ventricular outflow tract. Int J Cardiol. 2008. 126:e1–e3.
8. Satpathy R, Lynch J, Mohiuddin SM. Ventricular metastasis without atrial or caval involvement: a rare presentation. Echocardiography. 2008. 25:521–525.
9. Bradley SM, Bolling SF. Late renal cell carcinoma metastasis to the left ventricular outflow tract. Ann Thorac Surg. 1995. 60:204–206.
10. Canda AE, Kirkali Z. Current management of renal cell carcinoma and targeted therapy. Urol J. 2006. 3:1–14.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download