Abstract
Background and Objectives
We describe our experience with combined open and endovascular repair in patients who have aortic arch pathology.
Subjects and Methods
This study is a retrospective analysis of 7 patients who underwent combined open and endovascular repair for aortic arch pathology. Medical records and radiographic information were reviewed.
Results
A total of 7 consecutive patients (5 men, 71.4%) underwent thoracic stent graft implantation. The mean age was 59.9±16.7 years. The indication for endovascular repair was aneurysmal degeneration in 5 patients, and rupture or impending rupture in 2 patients. In all 7 cases, supra-aortic transposition of the great vessels was performed successfully. Stent graft implantation was achieved in all cases. Surgical exposure of the access vessel was necessary in 2 patients. A total of 9 stent grafts were implanted (3 stent grafts in one patient). The Seal thoracic and the Valiant endovascular stent graft were implanted in 6 patients and 1 patient, respectively. There were no post-procedure deaths or neurologic complications. In 2 patients, bleeding and injury of access vessel were noted after the procedure. Postoperative endoleak was noted in 1 patient. One patient died at 10 months after the procedure due to a newly developed ascending aortic dissection. No patients required secondary intervention during the follow-up period. The aortic diameter decreased in 4 patients. In 3 patients, including 1 patient with endoleak, there was no change in aortic diameter.
Aortic arch aneurysm or dissection repair, requiring cardiopulmonary bypass and hypothermic circulatory arrest, remains a surgical challenge with a high rate of mortality (7-17%) and neurologic complication (4-12%).1-3) Endovascular repair for thoracic and abdominal aortic aneurysm is associated with lower perioperative morbidity and mortality rates than conventional open repair, with similar early and midterm follow-up results.4-8) Recently, combined open and endovascular repair has emerged as an effective adjunct in the treatment of various pathologies of the aortic arch, especially in the high-risk patient not suitable for conventional open repair owing to cardiovascular or pulmonary comorbidities.9-13) In the current report, we describe our experience with combined open and endovascular repair in patients who had an aneurysm or dissection involving the aortic arch.
From December 2007 to October 2009, 16 patients were treated at our institution with endovascular repair for thoracic aortic disease. This study is a retrospective analysis of the 7 patients who underwent combined open and endovascular repair for aortic disease involving the aortic arch with/without proximal descending aorta. Patient selection for combined repair was based on the length of the proximal landing zone and comorbidities. Patient demographics and clinical risk factors are shown in Table 1. Diagnosis was confirmed by enhanced computed tomography scan angiography (CTA) and angiography in all cases. Patients in whom the distal extent of disease was confined to the thoracic aorta were included in this study. Cases of aortic trauma resulting in pseudoaneurysm or dissection were also included in the patient cohort. Our indications for endovascular repair were the following: 1) maximum aortic diameter ≥55 mm; 2) rapid aortic enlargement (≥10 mm per year); 3) clinical or radiographic evidence of rupture or impending rupture; 4) intractable chest pain, despite maximal medical therapy.
Medical records and radiographic information were reviewed to determine the operative indications, the repair technique, the peri-procedural complications and the outcomes. Technical success was defined as a successful stent graft deployment without death, conversion to open repair or diagnosis of endoleak before discharge. For each patient, the immediate post-procedural CTA was compared with the most recent CTA. The following parameters were recorded: presence of an endoleak and its nature, and overall maximal aortic diameter. Adverse clinical events (mortality, respiratory failure, malperfusion, bleeding, vascular injury, renal failure, stroke and paraplegia) occurring during the peri or post-procedural period and during follow-up were recorded.
For arterial access, preoperative evaluation was done by CTA scans to exclude major occlusive disease of the aortoiliac axis. These CT scans were also used as a tool to predict the required length of the intended proximal landing zone. As a prerequisite for successful stent graft placement, a proximal landing zone of at least 1.5 cm along the lesser curvature of the aortic arch was claimed. Furthermore, after supra-aortic transposition, an additional CTA scan was performed to reconfirm the effective length of the intended landing zone extension.
In all 7 cases, supra-aortic transposition of the great vessels was performed several days prior to endovascular stent grafting.
A standard approach through a skin incision parallel to the left clavicle was chosen. The left subclavian artery (LSCA) was divided at its origin at the level of the aortic arch. The vessel was guided dorsal to the left jugular vein, and an end-to-side anastomosis between the LSCA and the left common carotid artery (LCCA) was performed.
Through an upper hemisternotomy approach, all supra-aortic vessels were exposed. Prosthetic graft was used to connect the aorta to the brachiocephalic artery (BA), the LCCA and the LSCA because extensive mobilization of the supra-aortic vessels is not sufficient to accomplish tension free vascular transposition.
All procedures were performed in an angiography suite, under general or local anesthesia. The right or left common femoral artery (CFA) was used for access in all the cases to place the endovascular stent grafts. In the majority of patients, stent graft was deployed using a percutaneous approach. Post-procedure, a suture-mediated closure device (Perclose™, Abbott Laboratories, Illinois, IL, USA) was used for closure of the access site (Preclose technique).14) Surgical exposure of the access vessel was required in 2 patients who had a severely tortuous CFA. Drainage of cerebrospinal fluid was performed in patients who required extensive aortic coverage or had had previous aortic surgery. Stent graft deployment was routinely performed under hypotonic conditions (60 mmHg systolic pressure) by overpacing at 180 beats per minute. Stent grafts from the same manufacturer were used when a patient required multiple stent grafts. To achieve a satisfactory seal, devices were oversized in diameter by 15-20% in relation to the diameter of the proximal landing zone. If an endoleak occurred after stent graft implantation, ballooning was carried out to make the stent graft closely adhere to the blood vessel wall. If the endoleak was caused by stent displacement or angulation, an aortic extending stent graft was implanted.
Type I endoleaks were defined as attachment site leaks, type Ia at the proximal attachment site and type Ib at the distal attachment site. Type II endoleaks were defined as branch leaks without attachment site connection. Type III endoleaks were defined as junctional leaks between stent grafts.
A total of 7 consecutive patients (5 men, 71.4%) were implanted with a thoracic stent graft for the treatment of aortic disease involving the aortic arch. The mean age at intervention was 59.9±16.7 years (range, 28-76) and the mean time between the supra-aortic transposition and endovascular repair was 41.6±38.9 days (range, 6-119). The mean time between symptom onset and endovascular repair was 178.4±163.3 days (range, 48-430) (Table 1). Indications for endovascular repair were: aneurysmal degeneration in 5 cases (71.4%), rupture or impending rupture in 2 cases (28.6%).
In all 7 cases, supra-aortic transposition of the great vessels was performed prior to endovascular stent grafting. In 3 patients who had an aneurysm extending from the ascending aorta, Y-shaped bypass surgery with a prosthetic graft to connect the aorta to the BA, LCCA and LSCA was performed. In one patient, Y-shaped bypass surgery with a prosthetic graft connecting the aorta to the BA and LCCA was performed and followed by end-to-side anastomosis between the LCCA and LSCA (Fig. 1). In another patient who had two localized traumatic aortic dissections of the ascending aorta and the just distal LSCA, graft replacement was performed in the ascending aorta, followed by graft interposition between the aorta and the LCCA, and end-to-side anatomosis between the LCCA and the LSCA. In the remaining 2 cases, who had relatively limited aneurysms in the aortic arch, transposition of the LSCA onto the LCCA was performed (Table 2).
Stent graft implantation was achieved in all cases. As described above, surgical exposure of the access vessel was necessary in 2 patients. Two different stent graft systems were used. The Seal thoracic endovascular stent graft (S & G biotech, Seoul, Korea) was used in 6 patients and the 3 Valiant endovascular stent grafts (Medtronic Inc, Santa Rosa, CA, USA) were implanted in 1 patient who had a long aortic aneurysm (Table 3).
There were no post-procedure deaths or neurologic complications. In one patient who underwent the procedure via percutaneous approach, continuous bleeding at the access site was noticed even though a suture-mediated closure device was used. Right CFA injury was noted and surgical repair performed. In another patient, a huge hematoma was noted in the groin area after the procedure. CTA showed active bleeding from the right deep circumflex iliac artery, which was managed with coil embolization. There were no other postoperative complications. Postoperative endoleak was noted in 1 patient on the follow up CTA. Therefore, the procedure was considered technically successful in 6 cases (85.7%) (Table 4).
Clinical follow-up was available for all patients and the mean clinical follow-up period was 14.0±4.5 months (range, 4 to 23 months). One patient died at 10 months after the procedure due to a newly developed ascending aortic dissection. The patient had initially undergone stent graft deployment for type B aortic dissection. But type Ia endoleak was noted after the procedure. The follow-up CTA scan showed persistent endoleak and slightly progressed aortic dissection. Consequently, implantation of an additional stent graft was planned. After the LSCA-to-LCCA bypass surgery, the stent graft was deployed just proximal to the previous stent graft. Postprocedure, the type Ia endoleak disappeared and follow-up CTA showed decreased aortic diameter with complete thrombosis of the false lumen. At 10 months after the procedure, the patient came to the emergency room with sudden onset chest pain and loss of consciousness. The CTA scan showed newly developed aortic dissection of the ascending aorta and hemopericardium and the patient eventually expired. No patients required secondary intervention during the follow-up period.
Follow up CTA scan showed persistent endoleak without change of aortic diameter in the patient who had showed endoleak immediately post-procedure. The aortic diameter decreased in 4 patients. In 3 patients including 1 patient who had endoleak, there was no interval change of aortic diameter.
Conventional surgical repair for aortic arch pathology carries a high mortality and morbidity, with a particularly significant incidence of neurologic injury.1-3) Recent advances in stent graft technology have enhanced the management of diseases of the descending thoracic aorta by avoiding thoracotomy and expanded the group of patients eligible for treatment. Because of the complex anatomy of the aortic arch, however, repair of aortic arch pathology remains a significant endovascular challenge as it requires preservation of the supra-aortic great vessels during the procedure. Moreover, insufficient proximal landing zone for stent graft deployment is a major limitation of endovascular repair in most cases. Generally, a landing zone length of more than 1.5 cm is considered acceptable, although conflicting evidence exists in this regard.15-18)
On the other hand, a sufficient proximal landing zone can be achieved by transposition of the supra-aortic vessels. Importantly, this procedure, which avoids the need for cardiopulmonary bypass and aortic cross-clamping, may have advantages for high-risk patients.19) In addition, second-stage endovascular repair may be performed much sooner than a second open procedure for patients requiring a two-stage approach. Bergeron et al.20) reported results of combined open and endovascular repair for 15 aortic arch pathologies. The success rates of transposition of the great vessels and endovascular repair were 97% and 92%, respectively. In our study, supra-aortic transposition was successful in all patients and the technical success rate of endovascular repair was 85.7%. No deaths related to supra-aortic transposition occurred in our study, thereby emphasizing the safety of these procedures. In 2006, Saleh and Inglese21) successfully treated 15 aortic aneurysms using combined transposition of the supra-aortic great vessels and endovascular stent graft deployment. The success rate of these procedures was 100%. All stent grafts and bypass vessels were patent without endoleaks, stent displacement, or neurological deficits in the early postoperative period. One patient died 2 months postoperatively because of a pulmonary complication. In our study, except for one case in which endoleak was noted immediately after the procedure, all stent grafts and bypass vessels were patent without stent displacement in the early postoperative period. Each case in which endovascular stent grafting was performed after supra-aortic transposition of the great vessels was successful not only in effectively sealing or excluding the aortic arch lesions, but also in preserving the blood supply to the brain. There were no peri- or postoperative neurological complications, indicating that a combination of open supra-aortic transposition of the great vessels followed by endovascular stent grafting is an effective and safe method for treating aortic arch pathology.
Persistent primary endoleak was noted in one patient. Type I endoleak occurring after stent graft implantation can usually be treated by performing balloon angioplasty of the stent graft to make the stent tightly adhere to the blood vessel wall.22) This patient had diffuse extensive aortic aneurysm from the ascending aorta to the suprarenal abdominal aorta. After supra-aortic transposition (LSCA to LCCA anastomosis), a stent graft (Seal Thoracic 38×125 mm) was deployed. The problem, however, was that the proximal landing zone included aneurismal change with a diameter of around 36 mm. The stent graft was therefore not able to adhere to the aortic wall completely by balloon angioplasty. Immediate postoperative and follow-up CT scans showed persistent endoleak at the proximal site of the stent graft. To achieve sufficient proximal landing zone at the ascending aorta, Y-shaped bypass surgery with a prosthetic graft to connect the aorta to the supra-aortic vessels might have been helpful in this patient. Follow-up CT scan revealed no change in aortic diameter despite persistent endoleak, so secondary intervention was not indicated.
In one patient who underwent stent graft placement through a percutaneous approach, bleeding was not controlled at the access site after removal of the sheath. Emergent operation revealed the puncture site to be located around the bifurcation site of the superficial and deep femoral arteries, showing that the suture-mediated closure device had not worked properly. In this technique, care should be taken to puncture the CFA along its anterior aspect at least 1 cm proximal to the origin of the deep femoral artery. This can be confirmed by femoral angiography using an ipsilateral oblique projection.
In summary, the use of combined open and endovascular repair in the treatment of aortic arch pathology appears safe and effective at perioperative, postoperative and early midterm follow-up and offers several advantages over conventional repair, including the potential to offer therapy to patients who are not candidates for open repair and a shorter time between stages for patients requiring two-stage repair.
Our experience suggests that combined open and endovascular repair for aortic arch pathology is safe and effective, with few complications. In cases without sufficient landing zone for stent graft at the proximal end, complete or partial supraaortic transposition of the great vessels can be performed to ensure both cerebral blood supply and sufficient landing zone for the stent graft. Given the small population and relatively short follow-up period of our study, a much larger clinical study with a longer follow-up period may be warranted to further demonstrate the efficacy of this procedure.
Figures and Tables
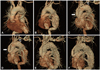
Fig. 1
Reconstructive three-dimensional computed tomography showed extensive aortic aneurysm involving the aortic arch and proximal descending thoracic aorta (A and B). Y-shaped bypass surgery with a prosthetic graft, to connect the aorta to the BA and the LCCA, was performed (white arrow) and followed by end-to-side anastomosis of the LCCA and LSCA (open arrow) (C and D). After supra-aortic transposition of the great vessels, 3 Valiant stent grafts were implanted from the ascending aorta to the descending thoracic aorta in a telescopic fashion to exclude extensive aortic aneurysm (E and F). BA: brachiocephalic artery, LCCA: left common carotid artery, LSCA: left subclavian artery.
References
1. Bachet J, Guilmet D, Goudot B, et al. Antegrade cerebral perfusion with cold blood: a 13-year experience. Ann Thorac Surg. 1999. 67:1874–1878. discussion 1891-4.
2. Harrington DK, Walker AS, Kaukuntla H, et al. Selective antegrade cerebral perfusion attenuates brain metabolic deficit in aortic arch surgery: a prospective randomized trial. Circulation. 2004. 110:11 Suppl 1. II231–II236.
3. Westaby S, Katsumata T, Vaccari G. Arch and descending aortic aneurysms: influence of perfusion technique on neurological outcome. Eur J Cardiothorac Surg. 1999. 15:180–185.
4. Gottardi R, Funovics M, Eggers N, et al. Supra-aortic transposition for combined vascular and endovascular repair of aortic arch pathology. Ann Thorac Surg. 2008. 86:1524–1529.
5. Joung B, Kang WC, Lee SH, et al. Favorable late outcome of endovascular abdominal aortic aneurysm repair. Korean Circ J. 2003. 33:797–804.
6. Kim BK, Park S, Ko YG, et al. Immediate and mid-term outcomes of the endovascular stent-graft treatment of abdominal aortic aneurysm. Korean Circ J. 2005. 35:583–590.
7. Stone DH, Brewster DC, Kwolek CJ, et al. Stent-graft versus open-surgical repair of the thoracic aorta: mid-term results. J Vasc Surg. 2006. 44:1188–1197.
8. Bavaria JE, Appoo JJ, Makaroun MS, Verter J, Yu ZF, Mitchell RS. Endovascular stent grafting versus open surgical repair of descending thoracic aortic aneurysms in low-risk patients: a multicenter comparative trial. J Thorac Cardiovasc Surg. 2007. 133:369–377.
9. Buth J, Penn O, Tielbeek A, Mersman M. Combined approach to stent-graft treatment of an aortic arch aneurysm. J Endovasc Surg. 1998. 5:329–332.
10. Czerny M, Fleck T, Zimpfer D, et al. Combined repair of an aortic arch aneurysm by sequential transposition of the supra-aortic branches and endovascular stent-graft placement. J Thorac Cardiovasc Surg. 2003. 126:916–918.
11. Czerny M, Zimpfer D, Fleck T, et al. Initial results after combined repair of aortic arch aneurysms by sequential transposition of the supra-aortic branches and consecutive endovascular stent-graft placement. Ann Thorac Surg. 2004. 78:1256–1260.
12. Dambrin C, Marcheix B, Hollington L, Rousseau H. Surgical treatment of an aortic arch aneurysm without cardio-pulmonary bypass: endovascular stent-grafting after extra-anatomic bypass of supra-aortic vessels. Eur J Cardiothorac Surg. 2005. 27:159–161.
13. Gottardi R, Seitelberger R, Zimpfer D, et al. An alternative approach in treating an aortic arch aneurysm with an anatomic variant by supraaortic reconstruction and stent-graft placement. J Vasc Surg. 2005. 42:357–360.
14. Lee WA, Brown MP, Nelson PR, Huber TS, Seeger JM. Midterm outcomes of femoral arteries after percutaneous endovascular aortic repair using the Preclose technique. J Vasc Surg. 2008. 47:919–923.
15. Antona C, Vanelli P, Petulla M, et al. Hybrid technique for total arch repair: aortic neck reshaping for endovascular-graft fixation. Ann Thorac Surg. 2007. 83:1158–1161.
16. Czerny M, Cejna M, Hutschala D, et al. Stent-graft placement in atherosclerotic descending thoracic aortic aneurysms: midterm results. J Endovasc Ther. 2004. 11:26–32.
17. Czerny M, Grimm M, Zimpfer D, et al. Results after endovascular stent graft placement in atherosclerotic aneurysms involving the descending aorta. Ann Thorac Surg. 2007. 83:450–455.
18. Wheatley GH, Gurbuz AT, Rodriguez-Lopez JA, et al. Midterm outcome in 158 consecutive Gore TAG thoracic endoprostheses: single center experience. Ann Thorac Surg. 2006. 81:1570–1577.
19. Hughes GC, Nienaber JJ, Bush EL, Daneshmand MA, McCann RL. Use of custom Dacron branch grafts for "hybrid" aortic debranching during endovascular repair of thoracic and thoracoabdominal aortic aneurysms. J Thorac Cardiovasc Surg. 2008. 136:21–28.
20. Bergeron P, Mangialardi N, Costa P, et al. Great vessel management for endovascular exclusion of aortic arch aneurysms and dissections. Eur J Vasc Endovasc Surg. 2006. 32:38–45.
21. Saleh HM, Inglese L. Combined surgical and endovascular treatment of aortic arch aneurysms. J Vasc Surg. 2006. 44:460–466.
22. Kato N, Shimono T, Hirano T, et al. Midterm results of stent-graft repair of acute and chronic aortic dissection with descending tear: the complication-specific approach. J Thorac Cardiovasc Surg. 2002. 124:306–312.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download