Abstract
Background and Objectives
As shown in previous studies, pentraxin 3 (PTX3) can be a useful inflammatory marker for metabolic syndrome and central obesity. Serum PTX3 levels are also an independent factor associated with visceral fat area. The aim of this study was to assess the role of PTX3 as an inflammatory maker in patients with central obesity undergoing primary percutaneous coronary intervention (PCI) following an ST-segment elevation myocardial infarction (STEMI).
Subjects and Methods
From December 2007 to June 2008, 40 subjects (mean age: 61±11 years, M : F=34 : 6) with STEMI who underwent primary PCI were enrolled. We determined waist circumference, waist/hip ratio, body mass index (BMI), and visceral and total fat area via fat computed tomography (FAT-CT), and compared them with serum PTX3 concentrations.
Results
The serum PTX3 concentration was closely related to FAT-CT-estimated visceral fat area (r=0.41, p<0.01) and total fat area (r=0.38, p=0.01), respectively. The serum PTX3 concentration was not related to waist circumference (r=0.27, p=0.20), waist circumference/hip ratio (r=0.25, p=0.16), BMI (r=0.04, p=0.80) and lipid profiles, respectively. Among the parameters determining metabolic syndrome, an increasing visceral fat area had the strongest association with PTX3 concentrations.
Arteriosclerosis has been shown to progress because of a persistent inflammatory reaction. Numerous studies have been conducted on the relationship between coronary artery diseases and the inflammatory reaction,1) with recent studies reporting that the inflammatory reaction is mutually associated with lipid metabolism and the metabolic syndrome.2-4) Markers of inflammatory reaction are also markers in the diagnosis and prognosis of cardiovascular diseases, and in particular have had practical applications in the diagnosis and prognosis of myocardial infarction, unstable angina, and heart failure. C-reactive protein (CRP), serum amyloid P, and pentraxin 3 (PTX3) are among well known inflammatory markers.5)6) CRP and PTX3 belong to the same PTX superfamily. Whereas CRP is a short PTX and is synthesized in the liver upon stimulation with primarily interleukin (IL)-6, PTX3 is a long PTX and is synthesized upon stimulation with diverse cytokines, such as IL-1 and tumor necrosis factor (TNF)-α, and it is specifically present in abundance in cardiac muscle.7-9) Because of the latter characteristic, PTX3 is reported to be a more specific marker than CRP in cardiovascular diseases, and studies have been conducted on its usefulness as a marker for early diagnosis and prognosis in acute myocardial infarction patients and as an inflammatory marker in chronic heart failure patients.10)11)
Previous studies have reported that PTX3 is useful as an inflammatory marker in metabolic syndrome patients, and that there is a correlation between PTX3 levels and abdominal obesity.12) A recent study found that in terms of type of abdominal obesity, PTX3 is closely associated with visceral fat tissues and it may also be associated with arteriosclerosis.13) However, studies on the correlation of PTX3 levels with metabolic syndrome or abdominal obesity in cardiovascular disease patients remain limited. Therefore, our study assessed whether there are associations among the concentration of serum PTX3, the metabolic syndrome, and abdominal obesity in myocardial infarction patients undergoing primary percutaneous coronary intervention (PCI) for ST-segment elevation myocardial infarction.
The study was conducted on 40 patients who had acute myocardial infarctions and were hospitalized via the emergency room at Uijeongbu Saint Mary's Hospital from December 2007 to June 2008 and treated with primary PCI. In all patients, the serum PTX3 level was measured during the procedure and after admission; blood tests, echocardiography, physical measurements, and fat computed tomography (FAT-CT) were also performed. Acute myocardial infarction was defined as ST segment changes corresponding to ischemia according to American College of Cardiology/American Heart Association (ACC/AHA) guidelines, and acute symptoms of myocardial ischemia or the elevation of myocardial enzyme levels. Cases with liver cirrhosis, chronic renal failure (plasma creatinine >2.0 mg/dL), autoimmune diseases, or acute inflammatory diseases were excluded. Metabolic syndrome was defined according to the standard suggested by the National Cholesterol Education Program, and the waist circumference was considered according to the recommended standard for the Asia-Pacific region; individuals with more than three of the following criteria were considered as having the metabolic syndrome: 1) waist circumference (male ≥90 cm, female ≥85 cm), 2) triglyceride level (≥150 mg/dL), 3) high density lipoprotein-cholesterol (HDL-C) level (male <40 mg/dL, female <50 mg/dL), 4) blood pressure (≥130/85 mmHg), and 5) fasting blood glucose level (≥100 mg/dL). Abdominal obesity was defined as visceral fat >103.8 cm2.14)
For patients diagnosed with acute myocardial infarction, coronary artery intervention was performed within 2 hours after arriving at the hospital and 10 mL of blood was collected from the femoral artery into ethylenediaminetetraacetic acid (EDTA) tubes, separated by centrifugation, and plasma was stored at -70℃. For the measurement of PTX3 levels, enzyme linked immuno sorbent assay (ELISA) plates were coated with PTX3 capture antibody (Alexis Biochemicals, ALX-804-464) at 4℃ overnight, blocked with 5% skim milk, and incubated with EDTA-plasma samples and the standard PTX3 protein at 37℃ for 2 hours. The samples and the standard protein were removed, washed, incubated with PTX3 detection antibody (Alexis Biochemicals, ALX-210-365B) at 37℃ for 1 hour and incubated with streptavidin-HRP (Zymed, 43-4323) at room temperature for 1 hour. The samples were incubated with TMB (tetramethyl benzidine; chromogenic method) (R&D, DY999) at room temperature for 20 minutes; after staining, 2N H2SO4 was added to stop the staining reaction and the plates were read. In addition, basic biochemical tests were performed.
Height and weight were measured by standardized devices and body mass index (BMI=kg/m2) was calculated. The waist circumference was measured in the middle of the bottom of lower rib and the pelvic iliac crest. The hip circumference was measured from the widest circumference of the greater trochanter area, and from this, the waist to hip ratio was calculated. Abdominal obesity was measured by FAT-CT (Somatom Plus, Siemens, Germany), and the visceral fat area, the subcutaneous fat area, and the total fat area were measured. The visceral-subcutaneous fat ratio was calculated by dividing the visceral fat area by the subcutaneous fat area.
FAT-CT scanned the 4th and 5th radius area within a 10 mm range. Dyes were not used. Areas with Hounsfield Units from -150 to -50 on CT were considered as fat, and the total fat, visceral fat and subcutaneous fat areas were automatically calculated.
Statistical data analysis was performed using the Statistical Package for the Social Sciences (SPSS) version 16.0 (SPSS Inc., Chicago, IL, USA), and statistical values are presented as a mean±standard error. Serum PTX3 concentration and continuous variables between the non-obese group and the obese group were compared using independent sample t-test, and the correlation of the continuous variables of serum PTX3 concentration and abdominal obesity was analyzed with the Pearson coefficient. Cases with a p less than 0.05 were considered to be statistically significantly different.
The study included 40 patients with a mean age of 61±11 years, 34 (85%) of whom were men. In terms of associated diseases, 15 (37.5%) of patients had hypertension, 11 (27.5%) had diabetes, 9 (22.5%) had hyperlipidemia, 17 (42.5%) met criteria for metabolic syndrome, 24 (60%) had abdominal obesity, and 28 (70 %) were smokers (Table 1).
Among these patients, 4 (10%) were on statins; nonetheless, the use of statins did not appear to affect levels of PTX3 (2.35±1.89 vs. 2.32±2.45, p=0.98), CRP (1.85±1.77 vs. 1.09±1.22, p<0.13) or serum lipids and it was not associated with abdominal obesity.
Among the 40 patients, serum PTX3 concentration correlated with the presence or absence of hypertension, diabetes, smoking, metabolic syndrome, or hyperlipidemia. When PTX3 levels were compared between those with or without each factor associated with the metabolic syndrome, there was no correlation with the waist circumference in male or female patients (male: r=0.19, p=0.29, female: r=0.48, p=0.34), BMI (r=0.04, p=0.80), triglycerides (r=0.07, p=0.96) HDL-C (r=0.15, p=0.35), blood pressure (SBP: r=0.81, p=0.62, DBP: r=0.15, p=0.37), or fasting blood glucose level (r=0.11, p=0.47). In addition, there was no correlation between PTX3 and CRP levels (r=0.24, p=0.14) (Table 2).
Abdominal obesity was defined by the visceral fat area measured by FAT-CT (>103.8 cm2), and serum PTX3 level and cardiovascular risk factors were compared according to the presence or absence of abdominal obesity.
Serum PTX3 concentrations tended to be increased in patients with a large visceral fat area (r=0.41, p<0.01) and total fat area (r=0.39, p=0.01); however, a correlation to subcutaneous fat area (r=0.25, p=0.12) was not detected (Fig. 1).
According to the presence or absence of abdominal obesity, in abdominal obesity patients, serum PTX3 concentration was significantly increased (3.00±2.61 vs. 1.33±0.81, p<0.01) (Fig. 2). Nevertheless, a difference of serum PTX3 concentration according to the presence or absence of metabolic syndrome was not seen (3.01±2.88 vs. 1.83±1.47, p=0.10).
When the CRP level (1.38±1.47 vs. 1.33±1.51, p=0.91), which is known to be associated with abdominal obesity and cholesterol values, was compared according to the presence or absence of abdominal obesity, there was no significant difference (Table 3).
Therefore, PTX3 levels measured in acute myocardial infarction patients correlated significantly to abdominal obesity measured by FAT-CT.
Our study suggests that among inflammatory markers whose levels are elevated in acute myocardial infarction patients, serum PTX3 is associated with metabolic syndrome and abdominal obesity.
Numerous studies have been conducted on the significance of serum PTX3 concentration, and in particular, on its role in the prognosis of acute myocardial infarction and chronic heart failure.10)11) Recently, a correlation between serum PTX3 concentration and metabolic syndrome was investigated, and was shown to be related to arteriosclerosis or dyslipidemia.15)
In our study, the PTX3 concentration and metabolic syndrome were not correlated, likely because of the small number of patients included and that they had had a myocardial infarction, which alone caused the elevation of the PTX3 level. Peri et al.10) have reported the role of PTX3 as an early marker of ischemic damage such as that in acute myocardial infarction, and showed that in these patients, the PTX3 level usually peaks 7.5 hours after admission to the intensive care unit (ICU), while the CRP level is elevated 24 hours after admission to the ICU. Our study revealed that within 2 hours after arrival to the emergency room, the serum PTX3 concentration was elevated to 2.33 ng/mL (normal value: <0.99 ng/mL). Latini et al.16) reported that in acute myocardial patients, serum PTX3 concentrations are superior to levels of CRP or other biochemical markers as a long-term prognosis marker.
CRP and serum PTX3 belong to the same PTX superfamily; nonetheless, CRP is a short PTX synthesized in the liver primarily in response to IL-6 stimulation, and serum PTX3 is a long PTX synthesized in response to stimulation by IL-1 and TNF-α, and it has been known to be specifically and abundantly present in cardiac muscle.7-9) It has been shown that, unlike CRP, serum PTX3 is rapidly induced by several inflammatory cytokines and primarily secreted from vascular endothelial cells.17) PTX3 secreted from cardiac vascular endothelial cells in response to several immunological signals18) is elevated not only in coronary artery diseases, but also in heart failure and has been reported to correlate with prognosis in heart failure.11)
Recent studies have reported that adipocytes induce PTX3 synthesis by secreting various inflammatory cytokines19) Alberti et al.13) have reported that serum PTX3 concentrations were higher in patients with visceral obesity than those with subcutaneous obesity. In our study, in acute myocardial infarction patients, subcutaneous fat and visceral fat were measured quantitatively by FAT-CT, and it was observed that increases in PTX3 concentration were significantly associated with increases in visceral fat and abdominal fat areas. Nevertheless, there was no correlation between CRP or serum lipid levels and obesity; a statistical association with PTX3 concentration was not seen either, which might be explained by the fact that acute myocardial infarctions were associated with elevated levels of CRP and serum lipid values. Similarly, PTX3 levels did not correlate with CRP levels and it has been shown that in acute myocardial infarction, their temporal and site distribution are different.10)
In acute myocardial patients, metabolic syndrome has been known to be a major risk factor for major cardiac events because the latter is associated with systemic inflammatory factors.20)21) In addition, Yusuf et al.22) have reported a close relationship between myocardial infarction and obesity; among obesity markers, the waist-hip circumference ratio was more important than BMI, which in particular emphasized the importance of visceral fat. In our study, similarly, 60% of patients had abdominal obesity; however, in cases with BMI ≥25 kg/m2, it was low, namely 25%, likely because even in patients with normal BMI, abdominal obesity was shown in many cases (Fig. 3). Kip et al.23) have reported that obesity itself is meaningful as a part of metabolic syndrome rather than as an independent risk factor for cardiovascular diseases. Therefore, abdominal obesity should be further emphasized as a risk factor for cardiovascular diseases, together with obesity.
The limitations of our study are that first, it was conducted on a small number of patients, and thus a correlation between serum PTX3 and metabolic syndrome could not be shown clearly. Second, it was conducted on myocardial infarction patients in a regional community hospital and thus it is difficult to generalize sociodemographic factors. Third, it was conducted on acute myocardial patients and thus the effects of medications such as statins could not be distinguished, and the precise relationship between serum lipid levels which is usually elevated in obese patients or CRP and abdominal obesity associated with myocardial infarction could not be assessed.
In addition, long-term follow-up is ongoing to ascertain whether high serum PTX3 concentration is associated with abdominal obesity and thus correlates with long-term clinical cardiovascular complications.
Our study documents that in acute myocardial infarction patients, the elavation of serum PTX3 concentration is not only due to cardiovascular disease, but also to abdominal visceral obesity as measured by FAT-CT. Therefore, the elevation of serum PTX3 concentration could be used not only in the diagnosis and prognosis of cardiovascular diseases, but also as a marker predicting abdominal visceral obesity in acute myocardial infarction patients.
Figures and Tables
 | Fig. 1The relationship between serum pentraxine 3 concentration and fat area of 3 groups. A: total. B: visceral. C: subcutaneous. |
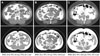 | Fig. 3Images demonstrate our method for determining abdominal fat distribution on a CT scan obtained at the umbilicus. The white areas demonstrated on upper images were regarded as visceral fat tissue, and the white areas on lower images were regarded as total fat tissue (visceral fat and subcutaneous fat). (A) is the CT scan in patient with normal BMI and normal VFA, (B) in patient with normal BMI and central obesity, and (C) in patient with increased BMI and central obesity. BMI: body mass index, WC: waist circumference, VFA: visceral fat area. |
References
1. Lusis AJ. Atherosclerosis. Nature. 2000. 407:233–241.
2. Noh HJ, Kwon NH, Joo SB. Severity of coronary atherosclerosis: influence of metabolic syndrome risk factor clustering and hs-CRP. Korean Circ J. 2006. 36:802–808.
3. Rattazi M, Faggin E, Bertipaglia B, Pauletto P. Innate immunity and atherogenesis. Lupus. 2005. 14:747–751.
4. Hansson GK, Robertson AL, Soderberg-Naucler C. Inflammation and atherosclerosis. Annu Rev Pathol. 2006. 1:297–329.
5. Koo BK, Choi DH, Ryu SK, et al. Role of inflammation on coronary artery disease in Koreans. Korean Circ J. 2002. 32:988–995.
6. Kim TI, Chae SC, Yang DH, Shin SC, et al. Short-term prognostic value of CRP in the patients with acute coronary syndrome. Korean Circ J. 2000. 30:1387–1394.
7. Mantovani A, Garlanda C, Bottazzi B. Pentraxin 3, a non-redundant soluble pattern recognition receptor involved in innate immunity. Vaccine. 2003. 21:Suppl 2. S43–S47.
8. Mantovani A, Garlanda C, Bottazzi B, et al. The long pentraxin PTX3 in vascular pathology. Vascul Pharmacol. 2006. 45:326–330.
9. Napoleone E, Di Santo A, Bastone A, et al. Long pentraxin PTX3 upregulates tissue factor expression in human endothelial cells: a novel link between vascular inflammation and clotting activation. Arterioscler Thromb Vasc Biol. 2002. 22:782–787.
10. Peri G, Introna M, Corradi D, et al. PTX3, A prototypical long pentraxin, is an early indicator of acute myocardial infarction in humans. Circulation. 2000. 102:636–641.
11. Suzuki S, Takeishi Y, Niizeki T, et al. Pentraxin 3, a new marker for vascular inflammation, predicts adverse clinical outcomes in patients with heart failure. Am Heart J. 2008. 155:75–81.
12. Moon DK, Lee DH, Youn HJ, et al. Pentraxin 3 as an inflammatory marker in subjects with metabolic syndrome. Korean J Med. 2007. 451S. Abstract.
13. Alberti L, Gilardini L, Zulian A, et al. Expression of long pentraxin PTX3 in human adipose tissue and its relation with cardiovascular risk factors. Atherosclerosis. 2009. 202:455–460.
14. Kim JA, Choi CJ, Yum KS. Cut-off values of visceral fat area and waist circumference: diagnostic criteria for abdominal obesity in a Korean population. J Korean Med Sci. 2006. 21:1048–1053.
15. Zenetti M, Bosutti A, Ferreira C, et al. Circulating pentraxin 3 levels are higher in metabolic syndrome with subclinical atherosclerosis: evidence for association with atherogenic lipid profile. Clin Exp Med. 2009. 9:243–248.
16. Latini R, Maggioni AP, Peri G, et al. Prognostic significance of the long pentraxin PTX3 in acute myocardial infarction. Circulation. 2004. 110:2349–2354.
17. Introna M, Alles VV, Castellano M, et al. Cloning of mouse PTX3, a new marker of the pentraxin gene family expressed at extrahepatic sites. Blood. 1996. 87:1862–1872.
18. Presta M, Camozzi M, Salvatori G, Rusnati M. Role of the soluble pattern recognition receptor PTX3 in vascular biology. J Cell Mol Med. 2007. 11:723–738.
19. Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006. 6:772–783.
20. Takeno M, Yasuda S, Otsuka Y, et al. Impact of metabolic syndrome on the long-term survival of patients with acute myocardial infarction-potential association with C-reactive protein. Circ J. 2008. 72:415–419.
21. Clavijo LC, Pinto TL, Kuchulakanti PK, et al. Metabolic syndrome in patients with acute myocardial infarction is associated with increased infarct size and in-hospital complications. Cardiovasc Rrevasc Med. 2006. 7:7–11.
22. Yusuf S, Hawken S, Ounpuu S, et al. Obesity and the risk of myocardial infarction in 27000 participants from 52 countries: a case-control study. Lancet. 2005. 366:1640–1649.
23. Kip KE, Marroquin OC, Kelley DE, et al. Clinical importance of obesity versus the metabolic syndrome in cardiovascular risk in women. Circulation. 2004. 109:706–713.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download