Abstract
Apoptosis is a tightly regulated, cell deletion process that plays an important role in various cardiovascular diseases, such as myocardial infarction, reperfusion injury, and heart failure. Since cardiomyocyte loss is the most important determinant of patient morbidity and mortality, fully understanding the regulatory mechanisms of apoptotic signaling is crucial. In fact, the inhibition of cardiac apoptosis holds promise as an effective therapeutic strategy for cardiovascular diseases. Caspase, a critical enzyme in the induction and execution of apoptosis, has been the main potential target for achieving anti-apoptotic therapy. Studies suggest, however, that a caspase-independent pathway may also play an important role in cardiac apoptosis, although the mechanism and potential significance of caspase-independent apoptosis in the heart remain poorly understood. Herein we discuss the role of apoptosis in various cardiovascular diseases, provide an update on current knowledge about the molecular mechanisms that govern apoptosis, and discuss the clinical implications of anti-apoptotic therapies.
Heart disease is the leading cause of morbidity and mortality in the developed world. Apoptosis, a highly regulated cell death process, plays an important role in numerous pathologic conditions involving the heart,1)2) and the inhibition of apoptosis is emerging as a potential therapeutic strategy. This review provides an overview of the evidence for apoptosis in cardiovascular disease, discusses the molecular pathways that may be involved, and reviews the clinical implications.
Apoptosis has been shown to be involved in both acute and chronic loss of cardiomyocytes in myocardial infarction, ischemic heart disease, reperfusion injury, various forms of cardiomyopathy, and the development of both acute and chronic heart failure.3-5) Animal and human studies have demonstrated that apoptosis is present in the border zone of the infarcted myocardium in the early phase, confirming the important role of apoptosis in acute myocardial loss after myocardial infarction.6)
Further studies showing that apoptosis is also present months later suggest that apoptosis may also play a role in remodeling and in the subsequent development of heart failure.7) Since cardiomyocyte loss is the most important determinant of morbidity and mortality after myocardial infarction, preventing cardiomyocyte loss becomes a critical issue in the management of myocardial infarction. A better understanding of the regulatory mechanisms of apoptotic signaling is crucial in devising such strategies.
In contrast to acute myocardial injury, the pathogenesis of chronic heart failure is characterized by the progressive loss of cardiomyocytes evolving over months to years. Numerous studies involving human and animal models of heart failure suggest that apoptosis may be an important contributor to cardiomyocyte loss in the setting of heart failure.3) However, since the prevalence of apoptosis is very low in most forms of chronic heart failure (usually <0.1% terminal deoxynucleotidyl transferase dUTP nick end labeling-positive cells), whether or not apoptosis significantly contributes to the pathogenesis of heart failure or is an epiphenomenon associated with end-stage heart failure is still debated.5) Nevertheless, even at a very low level, the contribution of apoptosis over months to years is likely to prove clinically significant in patients with chronic heart failure. The current dilemmas are what forms of cell death (apoptotic vs. non-apoptotic) predominate in chronic heart disease, and whether or not the inhibition of cell death in the form of chronic inhibition therapy will prove beneficial in blocking the progression of clinical heart failure.
Over the last two decades, much work has been done to identify and elucidate the molecular mechanisms that regulate and execute apoptosis. These studies have shown that apoptosis is a tightly regulated, cell death process that involves close interactions among various pro- and anti-apoptotic molecules. It is generally agreed that apoptosis cannot be strictly identified by only one or two characteristics. In fact, a number of different types of mechanisms have been identified and characterized, such as intrinsic vs. extrinsic pathway or caspase-dependent vs. caspase-independent apoptosis.
The caspases are a family of cysteine proteases that cleave target proteins at specific aspartate residues.8)9) The caspases are produced as zymogens that are activated after cleavage of their prodomains.8) Caspases are grouped based on structure and function. Initiator caspases possess a long prodomain with a functionally important interacting domain. Caspase-9 and -8 are examples of initiator caspases, which act upstream to initiate and regulate apoptosis, and downstream to activate effector caspases. In comparison, effector caspases, such as caspase-3, are characterized by short prodomains, and generally depend on initiator caspases for activation. Studies show that homologous deletions of specific caspases most often cause tissue-specific or stimulus-dependent effects, rather than a global suppression of cell death.10) These findings suggest that distinct sets of caspases may be involved in specific apoptotic pathways, and they likely act in a tissue-specific manner. In general, caspase-mediated apoptosis occurs either by extrinsic (involving death receptors) or intrinsic (mitochondria-mediated) pathways (Fig. 1).11-16) These two pathways usually converge on a common effector caspase, such as caspase-3, to execute the final morphologic and biochemical alterations that are characteristic of apoptosis.8)
The death receptor-mediated pathway is initiated by the binding of a death ligand {e.g., Fas ligand (FasL) or tumor necrosis factor-alpha (TNF-α)} to a membrane-bound death receptor (e.g., Fas or TNF-α receptor).14) This interaction leads to the recruitment of a death domain {e.g., Fas-associated death domain (FADD)}, which activates caspase-8 followed by the downstream effector caspases. Several studies suggest that the extrinsic apoptotic pathway has an important pathophysiologic role in the pathogenesis of heart failure.17-23) The importance of FADD, for example, has been demonstrated by gene knockout, which causes embryonic lethality resulting from heart failure and abdominal hemorrhage.17) Components of the death receptor-mediated apoptotic pathway are up-regulated in cardiomyocytes during myocarditis, particularly in immune-mediated cardiomyopathy.18) In human immunodeficiency virus cardiomyopathy, both death receptor- and mitochondria-mediated apoptosis pathways are involved in the apoptosis of cardiomyocytes.19) In addition, several studies have reported that failing human myocardium expresses high levels of TNF-α,20)21) and transgenic mice overexpressing cardiac-specific TNF-α develop dilated cardiomyopathy.22)23) These data suggest that increased levels of TNF-α are detrimental to the heart by activation of the death receptor pathway.
The Fas pathway may be an important mediator of cardiomyocyte apoptosis during ischemia/reperfusion (I/R) in vivo. Various knockouts of the death receptor pathway have been shown to improve cardiac function after I/R injury by inhibiting apoptosis. Mice lacking Fas exhibit reduction in infarct size after I/R.24) However, the heart has high levels of death receptor pathway inhibitors, such as apoptosis repressor with caspase recruitment domain (ARC) and FLICE-inhibitory protein. Indeed, cardiac-specific overexpression of FasL does not cause increased cardiomyocyte apoptosis,23)25) and TNF receptor 1 or 2 knockout in mice does not affect infarct size after coronary artery ligation.26) These findings suggest that although the death receptor-mediated pathway could be important in certain situations (e.g., autoimmune-mediated heart failure), the role of the death receptor-mediated pathway in myocardial infarction or I/R is unclear.
Mitochondria constitute approximately 30% of the cell volume in cardiomyocytes, and play an essential role by generating adenosine triphosphate (ATP) for cellular function. However, upon apoptotic stimulation, such as oxidative stress and serum deprivation, mitochondria become a critical organelle in the initiation of cell death. In the mitochondria-mediated or intrinsic apoptotic pathway, an apoptotic insult induces the mitochondria to release cytochrome c into the cytosol.11) There mitochondria forms an activation complex, the apoptosome, with apoptotic protein activating factor-1 (Apaf-1) and caspase-9.12)27) Apoptosome formation results in the autoprocessing of caspase-9, as well as the activation of downstream caspases, such as caspase-3.12)15)
The regulation of the release of apoptotic factors, such as cytochrome c from mitochondria, is modulated by the Bcl-2 family of proteins. The Bcl-2 family of proteins can be categorized as either anti-apoptotic (e.g., Bcl-2 and Bcl-xL) or pro-apoptotic (e.g., Bad, Bak, and Bax).28)29) One of the pro-apoptotic members, Bcl-2-interacting protein (Bid), may regulate the interaction between death receptor and mitochondrial pathways. Bid is usually located in the cytosol, but when it is cleaved to 'truncated Bid' (tBid) by activated caspase-8, Bid translocates to the mitochondria and regulates cytochrome c release.30) The protective role of anti-apoptotic Bcl-2 in the heart is demonstrated by the fact that cardiac-specific overexpression of Bcl-2 significantly reduces infarct size after I/R.31)32) Deleting pro-apoptotic Bax also results in reduced infarct size and improved function after experimental myocardial infarction.33)
Additional regulatory mechanisms of caspase-mediated pathways involve caspase inhibitors. Inhibitor of apoptosis proteins (IAPs) are prototypical inhibitors of caspases that block caspase function, usually by directly binding to the caspases.34) Cardiac-specific overexpression of cIAP2 reduces infarct size after I/R in isolated perfused hearts.35) Another important caspase inhibitor is ARC, which is found in high levels in skeletal muscle and heart.36)37) ARC interacts with upstream caspases and has been shown to block caspase-2 and -8, as well as cytochrome c release. The overexpression of ARC decreases infarct size after I/R.38) We have also identified other anti-apoptotic factors, such as HS-1 associated protein-1 (HAX-1), which acts by directly interacting with pro-caspase-9, and prevents its activation.39)
Although caspase activation is most likely the predominant mechanism in the induction of apoptosis, accumulating evidence demonstrates that apoptosis may also be mediated by mechanisms that do not involve caspases.40-42) The so-called caspase-independent pathways involve the release of apoptotic factors, such as apoptosis inducing factor (AIF), from mitochondria to the cytosol followed by translocation to the nucleus, where they cause deoxyribonucleic acid (DNA) fragmentation without concurrent caspase activation.43)44) In contrast to caspase-mediated apoptosis, which is characterized by an oligonucleosomal DNA fragmentation in multiples of -200 bp with an advanced chromatin condensation pattern, caspase-independent apoptosis is characterized by large scale DNA fragmentation (-50 kbp) with an early chromatin condensation pattern.41)42) The potential importance of caspase-independent pathways in the heart is highlighted by the fact that cardiomyocytes contain high levels of endogenous caspase inhibitors, thereby making them relatively resistant to caspase-dependent apoptosis.40) Thus, the role of caspase-independent apoptosis may be amplified in the heart.
The best documented example of caspase-independent apoptosis involves AIF,41-43)45)46) AIF is a flavoprotein localized in the mitochondrial intermembrane space and is required for oxidative phosphorylation.47) Upon apoptotic stimulation, AIF is released into the cytosol and translocates into the nucleus to induce DNA fragmentation without caspase activation.41) This notion is supported by the fact that microinjection of AIF into cells induces apoptotic changes, such as chromatin condensation, that are not blocked by a caspase inhibitor (zVAD.fmk),41)48) In the heart, AIF has been implicated in apoptosis induced by oxidative stress, ischemia reperfusion, and heart failure in vitro and in vivo.49)50) AIF also accumulates in the cytosolic and nuclear fractions of the heart following I/R.51) We have demonstrated significant activation of caspase-independent apoptosis in the Dahl salt-sensitive rat model of heart failure.52) We have also recently shown that AIF-induced apoptosis is activated in cardiomyocytes, especially in hypertrophic cardiomyocytes.53)
Despite its pro-apoptotic function, AIF has also been shown to possess an essential pro-survival function. Homozygous AIF knockout in a mouse is lethal to embryos,54) and the Harlequin (Hq) mouse, which expresses 10-20% of normal AIF levels, is prone to increased damage from I/R injury.55) In addition, a mouse with cardiac and skeletal muscle-specific knockout of AIF develops severe dilated cardiomyopathy and skeletal atrophy accompanied by defective mitochondrial respiratory activity.56) How, then, is AIF able to function as both a survival and a death-inducing factor? An elegant study by Cheung et al.57) using gene-targeted mice with various AIF mutants demonstrated that AIF is required for cell survival and normal mitochondrial respiration in neurons. On the other hand, during apoptotic stimulation, the pro-apoptotic function of AIF is recognized when AIF is released from mitochondria and translocates to the nucleus, where it promotes DNA damage.
Other caspase-independent apoptotic effectors have been demonstrated, including endonuclease G (Endo G), serine protease high temperature requirement protein A2 (HtrA2/Omi), and Bnip3. Endo G, a conserved nuclease, is involved in mitochondrial DNA replication with important roles in recombination and repair. Similar to AIF, Endo G translocates from the mitochondria to the nucleus during apoptosis and induces DNA fragmentation independent of caspases.58-60) Endo G and truncated AIF become the essential mediators of apoptosis in a caspase-independent manner in cardiomyocytes.61) Interestingly, Endo G null mice, however, do not have any obvious defects in development or in the regulation of apoptosis.58)59) HtrA2/Omi, a mitochondrial serine protease with pro-apoptotic properties, may also contribute to caspase-independent apoptosis.62) There is evidence that HtrA2/Omi also translocates from the mitochondria to the cytosol during I/R to induce apoptosis. In heart, a specific HtrA2/Omi inhibitor, ucf-101, has also been shown to attenuate apoptosis and decrease infarct size.63)
The endoplasmic reticulum (ER) is responsible for the synthesis and folding of secreted proteins, as well as Ca2+ storage. Several studies have demonstrated a role for ER stress in the pathogenesis of diabetes and heart failure.64) Consistent with these observations, defective ER quality control in transgenic mice with mutant KDEL receptor (a receptor for ER chaperones) causes dilated cardiomyopathy,65) suggesting that apoptosis mediated by ER stress may be a significant contributor to cardiovascular disease. ER stress-induced cell death may occur via two different mechanisms. Under ER stress, activated caspase-12 activates caspase-3, leading to apoptosis.66) The second death-signaling pathway activated by ER stress is activation of a transcriptional program via up-regulation of the transcription factor, CHOP/GADD 153. CHOP activates the transcription of genes encoding pro-apoptotic proteins, including the BH3-only protein, Puma.67) Recently, it has been suggested that Puma is a critical component of ER stress-induced apoptosis in cardiac myocytes.68) The Bcl-2 proteins have been shown to localize to the ER, where they can regulate the levels of Ca2+ stored in the ER.69)
This review is focused on apoptotic cell death, but non-apoptotic mechanisms, such as necrosis and autophagy, are also important cell death processes in heart. Necrosis, which has often been viewed as an accidental and uncontrolled cell death process, might also be a highly orchestrated type of programmed cell death, such as apoptosis, and this subset of regulated necrosis is termed necroptosis.70) Unlike apoptosis or necrosis, autophagy is primarily involved in survival. Autophagy enables cells to dispose of cytoplasm and organelles by fusing vesicles containing cellular components and lysosomes.71) However, several studies have demonstrated that autophagy has features resembling apoptosis, including a possible association with the caspases and Bcl-2.72-75) We also recognize that there is considerable controversy at present around differentiating the various types of cell death. There may be a spectrum of different mechanisms, and which mode of cell death predominates depends on the specific type of apoptotic stimuli, the degree of insult, and the intracellular ATP concentration. These are important and controversial issues at this time, and further studies are needed to clarify these modes of cell death.
Since apoptosis is implicated in the pathogenesis of many different cardiovascular diseases, the inhibition of apoptosis promises to be an extraordinarily important target for therapeutic intervention. Even though the therapeutic targeting of apoptotic pathways has potential in the treatment of heart failure, several important questions remain to be answered. First, it has not been shown whether or not the inhibition of apoptosis can delay or prevent the development of heart failure. It is possible that inhibiting apoptosis may simply result in the activation of another mode of cell death, such as necrosis, which may have more deleterious effects on neighboring cells and ultimately a worse outcome. Although the early studies on animal models of heart failure have been encouraging, the long-term consequences of inhibiting apoptosis in the heart are not known. Second, the safety of anti-apoptotic therapy has not been rigorously tested. Apoptosis is needed for the normal functioning of various cell systems, such as the immune system, and an excessive inhibition of apoptosis is associated with lymphoma or autoimmune disorders. Therefore, the chronic systemic inhibition of apoptosis may have significant deleterious consequences in non-cardiac organs. Third, anti-apoptotic therapy for heart failure may not apply to all types of heart failure. The most ideal conditions for anti-apoptotic intervention, in our opinion, occur in transient and acute insults, such as reperfusion. During reperfusion, cardiomyocyte apoptosis occurs at a high rate during a defined time period; thus, a short treatment period may be highly effective. Moreover, a short therapeutic course has the additional benefit of minimizing the possible deleterious side effects arising in other organ systems.
It is clear that apoptosis plays a critical role in the pathogenesis of various cardiovascular diseases and the inhibition of apoptosis promises to be an extraordinarily important target for therapeutic intervention. However, more work is necessary to understand the molecular mechanisms that govern these processes, and the significance of apoptosis in heart failure. For example, although caspase inhibition has been shown to reduce the acute loss of myocardium in various animal models,76)77) caspase inhibition might not be completely effective in blocking apoptotic cell death.78) With the potentially significant contribution of caspase-independent apoptotic cell death in the heart, it is important to better define the role of the caspase-independent pathway in cardiac apoptosis at this time. Further work must be carried out in well-defined experimental frameworks that are tissue-targeted and time-specific, with clear quantitative end points. Only then will we be able to conduct meaningful human studies to answer the question of whether the inhibition of apoptosis in heart failure will translate into clinical benefit.
Figures and Tables
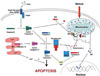 | Fig. 1Schematic diagram of apoptotic signaling. Apoptosis can be initiated by caspase-dependent or -independent mechanisms. In caspase-dependent mechanism, either death receptor or mitochondria (or both) are involved in initiation of apoptosis. In the caspase-independent mechanism, apoptotic factors, such as AIF, are released from the mitochondria, which trigger the apoptotic cascade. AIF: apoptosis inducing factor. |
Acknowledgments
This study was supported in part by grants from the National Institutes of Health RO1 HL091998 (P.M.K.), and the World Class University program from the Korean National Research Foundation (P.M.K.).
References
1. Saraste A, Voipio-Pulkki LM, Parvinen M, Pulkki K. Apoptosis in the heart. N Engl J Med. 1997. 336:1025–1026. discussion 6.
2. Kang PM, Izumo S. Apoptosis in heart: basic mechanisms and implications in cardiovascular diseases. Trends Mol Med. 2003. 9:177–182.
3. Kang PM, Izumo S. Apoptosis and heart failure: a critical review of the literature. Circ Res. 2000. 86:1107–1113.
4. Gottlieb RA, Burleson KO, Kloner RA, Babior BM, Engler RL. Reperfusion injury induces apoptosis in rabbit cardiomyocytes. J Clin Invest. 1994. 94:1621–1628.
5. Olivetti G, Abbi R, Quaini F, et al. Apoptosis in the failing human heart. N Engl J Med. 1997. 336:1131–1141.
6. Olivetti G, Quaini F, Sala R, et al. Acute myocardial infarction in humans is associated with activation of programmed myocyte cell death in the surviving portion of the heart. J Mol Cell Cardiol. 1996. 28:2005–2016.
7. Takemura G, Ohno M, Hayakawa Y, et al. Role of apoptosis in the disappearance of infiltrated and proliferated interstitial cells after myocardial infarction. Circ Res. 1998. 82:1130–1138.
8. Nicholson DW, Thornberry NA. Caspases: killer proteases. Trends Biochem Sci. 1997. 22:299–306.
9. Pop C, Salvesen GS. Human caspases: activation, specificity, and regulation. J Biol Chem. 2009. 284:21777–21781.
10. Zheng TS, Hunot S, Kuida K, Flavell RA. Caspase knockouts: matters of life and death. Cell Death Differ. 1999. 6:1043–1053.
11. Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996. 86:147–157.
12. Li P, Nijhawan D, Budihardjo I, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997. 91:479–489.
13. Zou H, Henzel WJ, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997. 90:405–413.
14. Nagata S. Apoptosis by death factor. Cell. 1997. 88:355–365.
15. Slee EA, Harte MT, Kluck RM, et al. Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J Cell Biol. 1999. 144:281–292.
16. Kang PM, Haunstetter A, Aoki H, Usheva A, Izumo S. Morphological and molecular characterization of adult cardiomyocyte apoptosis during hypoxia and reoxygenation. Circ Res. 2000. 87:118–125.
17. Yeh WC, Pompa JL, McCurrach ME, et al. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science. 1998. 279:1954–1958.
18. Ishiyama S, Hiroe M, Nishikawa T, et al. The Fas/Fas ligand system is involved in the pathogenesis of autoimmune myocarditis in rats. J Immunol. 1998. 161:4695–4701.
19. Twu C, Liu NQ, Popik W, et al. Cardiomyocytes undergo apoptosis in human immunodeficiency virus cardiomyopathy through mitochondrion-and death receptor-controlled pathways. Proc Natl Acad Sci U S A. 2002. 99:14386–14391.
20. Doyama K, Fujiwara H, Fukumoto M, et al. Tumour necrosis factor is expressed in cardiac tissues of patients with heart failure. Int J Cardiol. 1996. 54:217–225.
21. Torre-Amione G, Kapadia S, Lee J, et al. Tumor necrosis factor-alpha and tumor necrosis factor receptors in the failing human heart. Circulation. 1996. 93:704–711.
22. Bryant D, Becker L, Richardson J, et al. Cardiac failure in transgenic mice with myocardial expression of tumor necrosis factor-alpha. Circulation. 1998. 97:1375–1381.
23. Kubota T, McTiernan CF, Frye CS, et al. Dilated cardiomyopathy in transgenic mice with cardiac-specific overexpression of tumor necrosis factor-alpha. Circ Res. 1997. 81:627–635.
24. Lee P, Sata M, Lefer DJ, Factor SM, Walsh K, Kitsis RN. Fas pathway is a critical mediator of cardiac myocyte death and MI during ischemia-reperfusion in vivo. Am J Physiol Heart Circ Physiol. 2003. 284:H456–H463.
25. Nelson DP, Setser E, Hall DG, et al. Proinflammatory consequences of transgenic fas ligand expression in the heart. J Clin Invest. 2000. 105:1199–1208.
26. Kurrelmeyer KM, Michael LH, Baumgarten G, et al. Endogenous tumor necrosis factor protects the adult cardiac myocyte against ischemic-induced apoptosis in a murine model of acute myocardial infarction. Proc Natl Acad Sci U S A. 2000. 97:5456–5461.
27. Bao Q, Shi Y. Apoptosome: a platform for the activation of initiator caspases. Cell Death Differ. 2007. 14:56–65.
28. Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008. 9:47–59.
29. Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998. 281:1322–1326.
30. Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998. 94:491–501.
31. Brocheriou V, Hagege AA, Oubenaissa A, et al. Cardiac functional improvement by a human Bcl-2 transgene in a mouse model of ischemia/reperfusion injury. J Gene Med. 2000. 2:326–333.
32. Chen Z, Chua CC, Ho YS, Hamdy RC, Chua BH. Overexpression of Bcl-2 attenuates apoptosis and protects against myocardial I/R injury in transgenic mice. Am J Physiol Heart Circ Physiol. 2001. 280:H2313–H2320.
33. Hochhauser E, Cheporko Y, Yasovich N, et al. Bax deficiency reduces infarct size and improves long-term function after myocardial infarction. Cell Biochem Biophys. 2007. 47:11–20.
34. Salvesen GS, Dixit VM. Caspase activation: the induced-proximity model. Proc Natl Acad Sci U S A. 1999. 96:10964–10967.
35. Sun CK, Chang LT, Sheu JJ, et al. Losartan preserves integrity of cardiac gap junctions and PGC-1 alpha gene expression and prevents cellular apoptosis in remote area of left ventricular myocardium following acute myocardial infarction. Int Heart J. 2007. 48:533–546.
36. Ekhterae D, Lin Z, Lundberg MS, Crow MT, Brosius FC 3rd, Nunez G. ARC inhibits cytochrome c release from mitochondria and protects against hypoxia-induced apoptosis in heart-derived H9c2 cells. Circ Res. 1999. 85:e70–e77.
37. Koseki T, Inohara N, Chen S, Nunez G. ARC, an inhibitor of apoptosis expressed in skeletal muscle and heart that interacts selectively with caspases. Proc Natl Acad Sci U S A. 1998. 95:5156–5160.
38. Pyo JO, Nah J, Kim HJ, et al. Protection of cardiomyocytes from ischemic/hypoxic cell death via Drbp1 and pMe2GlyDH in cardio-specific ARC transgenic mice. J Biol Chem. 2008. 283:30707–30714.
39. Han Y, Chen YS, Liu Z, et al. Overexpression of HAX-1 protects cardiac myocytes from apoptosis through caspase-9 inhibition. Circ Res. 2006. 99:415–423.
40. Bae S, Yalamarti B, Kang PM. Role of caspase-independent apoptosis in cardiovascular diseases. Front Biosci. 2008. 13:2495–2503.
41. Lorenzo HK, Susin SA, Penninger J, Kroemer G. Apoptosis inducing factor (AIF): a phylogenetically old, caspase-independent effector of cell death. Cell Death Differ. 1999. 6:516–524.
42. Penninger JM, Kroemer G. Mitochondria, AIF and caspases: rivaling for cell death execution. Nat Cell Biol. 2003. 5:97–99.
43. Cande C, Cecconi F, Dessen P, Kroemer G. Apoptosis-inducing factor (AIF): key to the conserved caspase-independent pathways of cell death? J Cell Sci. 2002. 115:4727–4734.
44. Cregan SP, Dawson VL, Slack RS. Role of AIF in caspase-dependent and caspase-independent cell death. Oncogene. 2004. 23:2785–2796.
45. Cregan SP, Fortin A, MacLaurin JG, et al. Apoptosis-inducing factor is involved in the regulation of caspase-independent neuronal cell death. J Cell Biol. 2002. 158:507–517.
46. Susin SA, Lorenzo HK, Zamzami N, et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999. 397:441–446.
47. Vahsen N, Cande C, Briere JJ, et al. AIF deficiency compromises oxidative phosphorylation. EMBO J. 2004. 23:4679–4689.
48. Sharp TV, Wang HW, Koumi A, et al. K15 protein of Kaposi's sarcoma-associated herpesvirus is latently expressed and binds to HAX-1, a protein with antiapoptotic function. J Virol. 2002. 76:802–816.
49. Chen M, Zsengeller Z, Xiao CY, Szabo C. Mitochondrial-to-nuclear translocation of apoptosis-inducing factor in cardiac myocytes during oxidant stress: potential role of poly (ADP-ribose) polymerase-1. Cardiovasc Res. 2004. 63:682–688.
50. Xiao CY, Chen M, Zsengeller Z, et al. Poly (ADP-Ribose) polymerase promotes cardiac remodeling, contractile failure, and translocation of apoptosis-inducing factor in a murine experimental model of aortic banding and heart failure. J Pharmacol Exp Ther. 2005. 312:891–898.
51. Kim GT, Chun YS, Park JW, Kim MS. Role of apoptosis-inducing factor in myocardial cell death by ischemia-reperfusion. Biochem Biophys Res Commun. 2003. 309:619–624.
52. Siu PM, Bae S, Bodyak N, Rigor DL, Kang PM. Response of caspase-independent apoptotic factors to high salt diet-induced heart failure. J Mol Cell Cardiol. 2007. 42:678–686.
53. Choudhury S, Bae S, Kumar SR, et al. Role of AIF in cardiac apoptosis in hypertrophic cardiomyocytes from Dahl salt-sensitive rats. Cardiovasc Res. 2010. 85:28–37.
54. Joza N, Susin SA, Daugas E, et al. Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature. 2001. 410:549–554.
55. van Empel VP, Bertrand AT, van der Nagel R, et al. Downregulation of apoptosis-inducing factor in harlequin mutant mice sensitizes the myocardium to oxidative stress-related cell death and pressure overload-induced decompensation. Circ Res. 2005. 96:e92–e101.
56. Joza N, Oudit GY, Brown D, et al. Muscle-specific loss of apoptosis-inducing factor leads to mitochondrial dysfunction, skeletal muscle atrophy, and dilated cardiomyopathy. Mol Cell Biol. 2005. 25:10261–10272.
57. Cheung EC, Joza N, Steenaart NA, et al. Dissociating the dual roles of apoptosis-inducing factor in maintaining mitochondrial structure and apoptosis. EMBO J. 2006. 25:4061–4073.
58. David KK, Sasaki M, Yu SW, Dawson TM, Dawson VL. EndoG is dispensable in embryogenesis and apoptosis. Cell Death Differ. 2006. 13:1147–1155.
59. Irvine RA, Adachi N, Shibata DK, et al. Generation and characterization of endonuclease G null mice. Mol Cell Biol. 2005. 25:294–302.
60. Li LY, Luo X, Wang X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature. 2001. 412:95–99.
61. Bahi N, Zhang J, Llovera M, Ballester M, Comella JX, Sanchis D. Switch from caspase-dependent to caspase-independent death during heart development: essential role of endonuclease in ischemia-induced DNA processing on differentiated cardiomyocytes. J Biol Chem. 2006. 281:22943–22952.
62. Suzuki Y, Takahashi-Niki K, Akagi T, Hashikawa T, Takahashi R. Mitochondrial protease Omi/HtrA2 enhances caspase activation through multiple pathways. Cell Death Differ. 2004. 11:208–216.
63. Liu HR, Gao E, Hu A, et al. Role of Omi/HtrA2 in apoptotic cell death after myocardial ischemia and reperfusion. Circulation. 2005. 111:90–96.
64. Li Z, Zhang T, Dai H, et al. Involvement of endoplasmic reticulum stress in myocardial apoptosis of streptozocin-induced diabetic rats. J Clin Biochem Nutr. 2007. 41:58–67.
65. Hamada H, Suzuki M, Yuasa S, et al. Dilated cardiomyopathy caused by aberrant endoplasmic reticulum quality control in mutant KDEL receptor transgenic mice. Mol Cell Biol. 2004. 24:8007–8017.
66. Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006. 7:880–885.
67. Li J, Lee B, Lee AS. Endoplasmic reticulum stress-induced apoptosis: multiple pathways and activation of p53-up-regulated modulator of apoptosis (PUMA) and NOXA by p53. J Biol Chem. 2006. 281:7260–7270.
68. Nickson P, Toth A, Erhardt P. PUMA is critical for neonatal cardiomyocyte apoptosis induced by endoplasmic reticulum stress. Cardiovasc Res. 2007. 73:48–56.
69. Scorrano L, Oakes SA, Opferman JT, et al. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003. 300:135–139.
70. Degterev A, Huang Z, Boyce M, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005. 1:112–119.
71. Kim J, Klionsky DJ. Autophagy, cytoplasm-to-vacuole targeting pathway, and pexophagy in yeast and mammalian cells. Annu Rev Biochem. 2000. 69:303–342.
72. Gorski SM, Chittaranjan S, Pleasance ED, et al. A SAGE approach to discovery of genes involved in autophagic cell death. Curr Biol. 2003. 13:358–363.
73. Lee CY, Baehrecke EH. Steroid regulation of autophagic programmed cell death during development. Development. 2001. 128:1443–1455.
74. Liang XH, Jackson S, Seaman M, et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999. 402:672–676.
75. Berry DL, Baehrecke EH. Growth arrest and autophagy are required for salivary gland cell degradation in Drosophila. Cell. 2007. 131:1137–1148.
76. Laugwitz KL, Moretti A, Weig HJ, et al. Blocking caspase-activated apoptosis improves contractility in failing myocardium. Hum Gene Ther. 2001. 12:2051–2063.
77. Yaoita H, Ogawa K, Maehara K, Maruyama Y. Attenuation of ischemia/reperfusion injury in rats by a caspase inhibitor. Circulation. 1998. 97:276–281.
78. Okamura T, Miura T, Takemura G, et al. Effect of caspase inhibitors on myocardial infarct size and myocyte DNA fragmentation in the ischemia-reperfused rat heart. Cardiovasc Res. 2000. 45:642–650.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download