Abstract
Treatment of coronary chronic total occlusions (CTOs) remains a challenging obstacle, posing a considerable barrier to achieving successful complete revascularization. By nature of their complexity, percutaneous CTO interventions are associated with lower rates of procedural success, higher complication rates, greater radiation exposure and longer procedure times compared with non-CTO interventions. In the last few years, development in guidewires, devices and the emergence of new techniques from Japanese centers resulted in higher success rates in the hands of experienced operators. The impact of drug eluting stents on restenosis has improved long-term outcomes after successful recanalization. Successful revascularization is associated with improved long-term survival, reduced symptoms, improved left ventricular function and reduced need for coronary bypass surgery. This paper reviews the current devices and specialized crossing techniques of percutaneous intervention to relieve CTOs.
Chronic total occlusions (CTOs) are common finding in patients with coronary artery disease.1)2) Although one or more totally occluded coronary vessels are identified in diagnostic coronary angiograms, recanalization of a CTO is attempted in only 8% to 15% of patients undergoing percutaneous coronary intervention (PCI).3)4) The disparity between the frequency of CTO and percutaneous treatment underscores not only the technical and procedural complexities entailed in the management of this lesion subtype, but also the clinical uncertainties with regard to patient selection: which patients are likely to benefit from CTO revascularization? By nature of their complexity, PCI for CTO is associated with lower rates of procedural success, higher complication rates, greater radiation exposure and longer procedure times compared to interventions in non-CTO lesions. Despite these obstacles, the reported benefits of successful CTO PCI include symptom reduction, lower need for surgical revascularization and improvement in both ventricular function and survival.5-7)
Any definition of coronary CTO must consider the degree of lumen narrowing, antegrade blood flow and the age of the occlusion. Lesions can be classified as CTOs when there is Thrombolysis in Myocardial Infarction 0 flow within the occluded segment, and angiographic or clinical evidence or high likelihood of an occlusion duration ≥3 months. The temporal criterion used to define a CTO has varied widely in prior reports, typically ranging from >2 weeks to ≥3 months, which in part explains differences in inter-study lesion characteristics and the extent of procedural success.8)9) In general, a total occlusion of duration >3 months may be considered "chronic". The success rate of CTO PCI decreases with parameters such as occlusion duration, length and amount of calcification. Table 1 illustrates a list of lesion and patient-based parameters expected by consensus of the group to be predictive of procedural success.10) A predominance of favorable characteristics can lead highly experienced CTO-operators to achieve success rate >90%. This may drop to <60-70% in the presence of one or more unfavorable predictive factors.
Access route selection is dependent upon individual patient situation. For example, co-existing severe peripheral vascular disease may necessitate a radial approach, which, in turn, is subject to operator preference. The femoral artery is the usual and preferred vascular access used in PCI for CTO in most catheterization laboratories. Recently, indications for trans-radial PCI for CTO are expanding due to miniaturization of new devices, improvements in techniques and increasing experience in trans-radial PCI.11)12) Guiding catheter size is limited in the radial approach, but the radial artery can be readily used for contralateral injection (5 or 6 Fr diagnostic catheters).
Guiding catheter selection is the first key to success. It is important to provide maximum support for wire crossing and subsequent devices advancement, using coaxial orientation, good stability and optimal back-up force of the guiding catheter. The choice of guiding catheter shape is also dictated by personal experience and preference. For the right coronary artery (RCA) most operators would select routinely Amplatz Left (AL) 1 or 0.75, preferably with side holes in case of inadvertent deep intubation and small arterial diameters. For the so-called "shepard's crook" proximal RCAs an AL 1 or 2 may represent the best choice. In cases where there is very proximal occlusion or even lesion of the RCA, a Judkins right (JR) catheters felt to be the best option to avoid the risk of ostial damage, especially in 7 or 8 Fr to provide greater support, and use deep intubation or an anchor technique to obtain more support if required.13)14) For CTOs of the left coronary artery, modern extra backup shapes (e.g., XB, EBU, BL) are preferred to classical Judkins left catheters. For the left circumflex artery (LCx), particularly in the presence of a short left main, an AL 1 or 2 may provide better support and coaxial orientation. Either a large lumen guide of 7 Fr/8 Fr guide catheters offering greater passive support, or a large lumen 6 Fr/5 Fr guide with good active support after deep insertion into the proximal segment of the target artery are available options. The use of 6 Fr catheters is probably sufficient for more straightforward CTOs, while in the most complex lesions only 7 or 8 Fr guiding catheters are sufficiently large to advance 2 wires and 2 over-the-wire (OTW) catheters (for parallel wire technique). If an intravascular ultrasound (IVUS) guided technique is used, 8 Fr guides are mandatory to advance an IVUS catheter side-by-side with an OTW catheter.15) When selecting guiding catheter size, it should also be borne in mind that use of contrast media will be lower with 6 Fr catheters, compared to large 7 or 8 Fr catheters.
Simultaneous contralateral injection should be performed to evaluate the length of the lesion, as a target landmark of the PCI guidewire, and as a route of the retrograde approach.16)17) When the distal vessel is mainly filled by retrograde collaterals, or there are bridging collaterals originating near the occlusion that are likely to have their flow impaired after wire-catheter advancement, contralateral injection is mandatory from the start of the procedure. The contralateral approach can also be achieved by puncturing the same groin with a 4 Fr or 5 Fr diagnostic catheter, which may allow the procedure to be better tolerated. As bilateral angiography is paramount, the use of the two radial arteries, two femoral arteries or combination technique, using one radial and one femoral artery will probably be increasingly reported in the near future.
A floppy wire is often the best initial choice to negotiate the segment proximal to the occlusion and advance an OTW catheter up to the proximal stump, and then exchange to a stiffer dedicated wire. The more aggressive, dedicated CTO wires may cause damage to tortuous proximal segments before reaching the occlusion. Pathological examination of CTO lesions revealed that there were small vascular channels of 160 to 230 µm in diameter, which were connected to the proximal free space of the occlusions.18) These small vascular channels cannot be identified by fluoroscopic or cine-angiographic imaging, because their diameter is too small, and contrast dye cannot fill in the lumen. If the wire supported by the OTW catheter penetrates the occlusion, it may easily take advantage of the micro-channels that have access to the occluded segment, as frequently demonstrated in histological samples.19)
Because of poor feedback and lack of resistance offered to advancement, even in a subintimal position with polymer coated wires, careful attention should be paid with check angiography via contralateral injection to any distal intraluminal wire passage to avoid the creation of long subintimal tracks. The majority of operators suggests a step-up approach with wires of moderately increased stiffness at the beginning (Miracle 3), and subsequently switch to wires of greater stiffness and penetration ability, these often being tapered (Conquest pro wires). Others believe that the risk of initial dissection can be minimized, and the procedure can be shortened and simplified, if stiffer wires are selected initially to pass through a hard, occlusion cap.
Optimal shaping of the wire tip is essential for successful crossing. In general, a very small distal curve of approximately 1.0-1.5 mm, at 30-45 degrees (primary curve) is needed to penetrate the occlusion entry or distal cap and avoid creation of large, false lumen. This short tip bend can find the softest part within the CTO lesion. Normally, we make the second mild bend 3 to 6 mm proximal to the tip. This second bend can work as navigator, which be used to orientate the tip in the expected direction of the vessel take-off. Exactly how short an operator can make the tip bend is dependent on the length of the soldering of the spring coil at the tip of the guidewire. Normally, the length is more than 1 mm. Thus, the shortest tip bend one can make is 1 mm. However, Fielder X-treme is specially designed for the retrograde approach, to negotiate the corkscrew tortuosity of collateral small vessels. The length of soldering is less than 1 mm.20)
The use of an OTW system is essential in PCI for CTO lesions. The wires should be used in combination with an OTW microcatheter or balloon. This allows exchange of a floppy for a dedicated stiffer CTO wire, but also facilitates transmission of torque to the tip, and improves feedback. Furthermore, it allows adjustment of the primary and secondary curves throughout the procedure. The tip load of CTO guidewires can be changed according to the length extended from the tip of an OTW catheter (Fig. 1). If the operator wishes to increase stiffness, tip of the OTW system can be put more closely to the tip of the guidewire. Dedicated microcatheters may provide better tip flexibility than OTW balloons, and are useful for CTOs immediately distal to a bend (i.e., proximal LCX). Their larger inner lumen reduces friction during wire manipulation. It is also advantageous to have a radio-opaque marker at the very tip of the catheter, to avoid advancing too far inadvertently-a mistake seen with 1.50 mm balloons which possess a single mid-balloon marker. The disadvantage of microcatheters is that they are rarely able to cross the occlusion and must be exchanged over the wire for small balloons. Commonly used devices for CTO PCI are listed in Table 2.
In the "drilling" technique, the guidewire is rotated clockwise and counterclockwise while the tip is pushed modestly against the CTO lesion. The short tip bent of a guidewire can help to identify the loosest part of the lesion, and advance into the true lumen while avoiding the hardest plaque or vessel wall. In the "penetrating" technique, the operator aims at the target with the tip of the guidewire without clockwise and counterclockwise rotations. The drilling technique should be applied first, because of the risk of guidewire perforation, and its risk of causing intimal dissection is lower than that of the penetrating technique, and it works well in tortuous artery. The important tip in this technique is that one does not push the guidewire very hard. If the tip of the guidewire does not advance any more with gentle pushing-it is by far better to exchange for a stiffer wire, rather than pushing. If one pushes the wire hard, it will easily go into the subintimal space. Miracle series guidewires are better for the drilling technique than the Conquest series. However, if the proximal cap of the CTO lesion is very hard, one needs to penetrate it. For this purpose, the tapered-tip guidewires are adequate, since the penetration ability is dependent on tip stiffness, tip cross-sectional area and the slippery coating.21)22) Since the penetration power of the Conquest-Pro series guidewires is so high, these guidewires behave like needles. In order to use the penetrating technique, the target has to be clearly identified using multi-angle projections with bilateral simultaneous dye injection. One cannot rely on tip feel, over what one is able to see. The majority of CTO lesions can be passed through with Miracle 3. However, some lesions need very stiff guidewires, such as the Conquest-Pro 12, when the penetration technique is required.
When a wire enters a false channel, it is left in place in the dissection plane as marker, and a second guidewire is passed along the same path parallel to the first wire. The main pitfall is the occurrence of the two wires twisting with each other. In order to avoid wires twisting, usage of support catheter and appropriate wire selection and handling are essential. The PW technique has two main purposes: re-directing a wire inside the body of the CTO and puncturing distal CTO fibrous cap. The PW technique has been shown to increase success rate after a failed attempt with the conventional single wire technique.21)23) An important condition for using the PW technique is visualization of the distal true lumen, filled via collaterals on angiography. Indeed, visualization of the first guidewire and its relative position to the second guidewire, using orthogonal angiographic views, is necessary to ensure success of this technique. Contralateral injection is needed to visualize the distal true lumen, apart from cases with auto-collaterals. Of paramount importance is to adopt this technique before a large subintimal dissection occurs, as the chance of successful recanalization by the second guidewire decreases proportionally to the extent of subintimal dissection induced by the first guidewire. The second wire should be stiffer than the first one, and should have superior torquability. These characteristics allow better maneuver-ability of the second wire, and decrease the risk of wires twisting. The wire that we most commonly use as second wire is the Miracle 12 g, or the Cofianza Pro wire. As the second wire is advanced along the same path in parallel to the first wire, only limited rotation should be applied. At best, a 45-90° clockwise rotation followed by a similar degree counterclockwise rotation. Checks on multiple angiographic views should be performed to confirm the correct location of the second wire. The general benefits of the parallel wire technique include decreased fluoroscopy time, as one spends less time exchanging wires and crossing the lesion, reducing the amount of contrast medium used as one can confirm the position of the wire by looking at the first wire without the need for contrast injection.24)
IVUS can be used to improve the success rate of wiring during CTO-PCI. Failure of guide wire crossing is the commonest reason for failed CTO PCI procedure. Certain angiographic features, such as blunt stump with side branch, are negative indicator for successful outcomes. It is because of the difficulty in ascertaining the true course of wire entry and progression angiographically. In addition, the position of wire inside the CTO and the morphologic features of CTO play a significant role in the success of the procedure. IVUS use during CTO PCI can be very effective and helpful in completing the procedure successfully, as reported in the literature.25)26) There are mainly two types of IVUS-guided wiring techniques: IVUS-guided wiring at CTO entrance, and IVUS-guided penetration from subintimal space.
IVUS-guided wiring at CTO entrance: in general, contralateral angiography precisely helps to identify an entry of a CTO despite the absence of stump in total occlusion of a bifurcation lesion. However, the use of angiography sometimes fails to identify the entry point. In this situation, IVUS is useful to detect the entry point of the CTO if the branch is large enough to advance an IVUS catheter. Based on a series of IVUS images, the catheter is positioned at the occlusion in the main vessel and angiography is performed subsequently. The entry exists at the location of the IVUS transducer on angiography. Subsequently, the operator seeks a dimple at the entry with careful wire manipulation. This technique also helps to examine plaque hardness at the entrance.
IVUS-guided penetration from subintimal space: even when using the standard parallel wiring technique, the wires occasionally enlarge the subintimal space in difficult CTO procedures. Once the subintimal space expands beyond the distal end of CTO, the distal true lumen can hardly be seen in fluoroscopy. In these situations, we often have to abandon subsequent procedure, when angiographic guidance is only used. IVUS can differentiate a true lumen from a false lumen by identifying the presence of side branch (which arise only from the true lumen), intima and media (which surround the true lumen, but not the false lumen).27) IVUS can confirm when the guidewire has re-entered the true lumen from the false lumen (Fig. 2). Stiff wires (Conquest pro or Miracle 12) should be used as the second wire to penetrate the true channel. This technique sometimes requires balloon dilatation in the subintimal space to deliver the IVUS catheter, and therefore it should never be performed when wire perforation from the subintimal space is already detected. In addition, an 8 Fr-guiding catheter is indispensable to conduct simultaneous wiring under IVUS guidance. After successful wire crossing, multiple stenting is mandatory to fully cover the enlarged subintimal space. This technique could be one of the last alternatives in the antegrade approach, when standard wiring procedures fail in cases without a chance for the retrograde approach.
If the artery shows the bend at the CTO portion, the guidewire sometimes goes into the side branch distal to the proximal cap of the CTO lesion. In this situation, do not try to pull back and re-navigate the wire into the distal true lumen. One has to keep the first wire in position, and then take the second wire following the double-guidewire technique. In addition, one can take a 1.5 mm balloon, put it along the guidewire in the side branch and inflate it. This balloon dilatation will then break the proximal hard plaque, which enables the second wire to advance into the distal true lumen.
The retrograde approach via collateral channels in PCI for complex CTO can improve the success rate.28) Most interventionists will meet a few cases where the retrograde approach will provide unequaled advantages, but many are held back from taking the retrograde approach by lack of appropriate equipment and expertise. As a general rule, the retrograde approach should be used jointly with an antegrade approach. Only in a few cases when the retrograde approach should be used alone. The retrograde approach was first performed in the early 1990s. Since then, increased knowledge and practical experience as well as improved devices have led to refined strategies in its application. The most common reason for failure in percutaneous CTO treatment by an antegrade approach is the inability to successfully pass a guidewire across the lesion into the true lumen of the distal vessel. It is important to remember that the histopathologic features are different at the proximal and distal fibrous tissue. It is particularly dense at the proximal CTO part and loose at its distal end. When looked from the distal side during the retrograde approach, it has a concave shape, which prevents the wire from sliding in the subintimal space and facilitates successful penetration inside the CTO body.
The retrograde approach requires a channel between the occluded coronary artery and another patent coronary artery, which enables the wire to reach the distal CTO site in a retrograde manner. The inter-coronary channel can either be an epicardial collateral, a septal collateral or a bypass graft. One study evaluated collaterals by angiography showed that the anatomical course of the principal collateral is through septal connections in 44%, atrial epicardial connections in 32%, distal interarterial connections in 18% and bridging connections in 6%.29) Septal collaterals are the safer and most appropriate access route.30) Septal collaterals have either an intra-muscular course or run under the endocardium. Perforation of a septal collateral, which is surrounded by the myocardium, will in most cases stop bleeding after a long balloon inflation or even spontaneously. Due to the small size of septal collaterals, pre-dilatation with a small 1.25-mmsize balloon is necessary to allow its delivery up to the distal CTO site. The procedure requires placement of two guiding catheter: a first guiding catheter is placed in the vessel with the occlusion, and a second guiding catheter in the coronary artery from which the collateral channel arises. There are three points to be emphasized: 1) A super-selective contrast injection via microcatheter or OTW balloon catheter is mandatory to confirm the channel course as well as its continuous character; 2) the chosen inter-coronary channel should be located at least a few millimeters more distally than the distal CTO end. This allows hanging a co-axial position of the wire tip and the CTO; 3) specific materials are required because of the increased intra-arterial length the wire and balloon catheter need to pass (either a short 85-90 cm guiding catheter, or balloons with 150-155 cm long shaft).
Once the retrograde wire reached the distal CTO end, techniques via a bilateral approach {controlled antegrade and retrograde subintimal tracking (CART) technique; kissing wire technique; knuckle-wire technique} will be used in most cases. The retrograde single-wire technique can be used in a limited number of cases.
The CART technique is safe and the most successful bilateral approach in CTO intervention.31) The basic concept of the CART technique is to create a subintimal dissection with limited extension, only at the site of CTO. Procedure steps are shown and explained in Fig. 3. First a wire is advanced in an antegrade manner, from the proximal true lumen into the subintimal space at the CTO site. Next the retrograde wire is advanced from the distal true lumen into the CTO, then into the subintimal space at the CTO site. In order to enable the two wires to meet, a balloon is brought on the retrograde wire and inflated from the subintimal space to the distal end of the CTO. The two subintimal dissections tend to expand spontaneously toward each other and to connect. To keep the dissection open, it is important to leave the deflated balloon inside the subintimal space, then manipulate the antegrade wire targeting the deflated balloon. Nowadays, the reverse CART technique is a more popular method of the retrograde approach in Japan following development of stabilizing devices for retrograde wires, such as combination of Fielder XT wire and Corsair supporting catheter.
Several reasons will determine when to bring to an end attempts to cross the occlusion. Excessive dye use (typically around 600 mls in a non-diabetic patient with normal renal function; much less in patients at risk for contrast nephropathy) should strongly suggest to the operator that it is time to end the procedure. Procedural events, such as creation of a large false lumen, may render further wire manipulations futile: a second attempt at another time may have better chance of succeeding, if performed 4 or more weeks later to allow vessel wall healing. Wire manipulation may also cause intramural hematomas, resulting in loss of visualization of the distal vessel via collaterals. Under this circumstance, a second subsequent attempt can be considered. Last, but not least, excessive patient or operator fatigue may necessitate stopping the procedure, and possibly the need to plan for a second attempt. Persistent subintimal contrast staining is not necessarily a reason to stop: a parallel wire may still be successful. A second attempt after a failed CTO is successful in >50% of patients, especially when the mode of failure is understood and a feasible alternative approach has been formulated, including a possible retrograde approach.
By nature of their complexity, percutaneous revascularization of coronary CTO is associated with lower rates of procedural success, higher complication rates, greater radiation exposure, and longer procedure times. Despite these obstacles, reported benefits of successful CTO PCI include symptom reduction and improvement in both ventricular function and survival. Throughout the recent evolution of both equipment and techniques, percutaneous revascularization of coronary CTO remains a formidable endeavor. Ongoing observations of the benefits of CTO PCI, enhanced by data from randomized comparative trials, and embracing novel yet validated measures, promise continued interest in improving the devices and the techniques. A higher success rate is obtained through harmonious bland of conventional stiff wires and crossing techniques in CTO-PCI.
Figures and Tables
Fig. 2
The concept of IVUS-guided penetration technique: from false lumen to true lumen. IVUS: intravascular ultrasound.
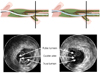
Fig. 3
Schema of different steps of the CART technique. CART: controlled antegrade and retrograde subintimal tracking.

References
1. Park CS, Kim HY, Park HJ, et al. Clinical, electrocardiographic, and procedural characteristics of patients with coronary chronic total occlusions. Korean Circ J. 2009. 39:111–115.
2. Christofferson RD, Lehmann KG, Martin GV, Every N, Caldwell JH, Kapadia SR. Effect of chronic total coronary occlusion on treatment strategy. Am J Cardiol. 2005. 95:1088–1091.
3. Anderson HV, Shaw RE, Brindis RG, et al. A contemporary overview of percutaneous coronary interventions: the American College of Cardiology-National Cardiovascular Data Registry (ACC-NCDR). J Am Coll Cardiol. 2002. 39:1096–1103.
4. Williams DO, Holubkov R, Yeh W, et al. Percutaneous coronary intervention in the current era compared with 1985-1986: the National Heart, Lung, and Blood Institute Registries. Circulation. 2000. 102:2945–2951.
5. Sirnes PA, Myreng Y, Molstad P, Bonarjee V, Golf S. Improvement in left ventricular ejection fraction and wall motion after successful recanalization of chronic coronary occlusions. Eur Heart J. 1998. 19:273–281.
6. Suero JA, Marso SP, Jones PG, et al. Procedural outcomes and long-term survival among patients undergoing percutaneous coronary intervention of a chronic total occlusion in native coronary arteries: a 20-year experience. J Am Coll Cardiol. 2001. 38:409–414.
7. Olivari Z, Rubartelli P, Piscione F, et al. Immediate results and one-year clinical outcome after percutaneous coronary interventions in chronic total occlusions: data from a multicenter, prospective, observational study (TOAST-GISE). J Am Coll Cardiol. 2003. 41:1672–1678.
8. Werner GS, Emig U, Mutschke O, Schwarz G, Bahrmann P, Figulla HR. Regression of collateral function after recanalization of chronic total coronary occlusions: a serial assessment by intracoronary pressure and Doppler recordings. Circulation. 2003. 108:2877–2882.
9. Zidar FJ, Kaplan BM, O'Neill WW, et al. Prospective, randomized trial of prolonged intracoronary urokinase infusion for chronic total occlusions in native coronary arteries. J Am Coll Cardiol. 1996. 27:1406–1412.
10. Di Mario C, Werner GS, Sianos G, et al. European perspective in the recanalisation of Chronic Total Occlusions (CTO): consensus document from the EuroCTO Club. EuroIntervention. 2007. 3:30–43.
11. Rathore S, Hakeem A, Pauriah M, Roberts E, Beaumont A, Morris JL. A comparison of the transradial and the transfemoral approach in chronic total occlusion percutaneous coronary intervention. Catheter Cardiovasc Interv. 2009. 73:883–887.
12. Kim JY, Yoon JH, Jung HS, et al. The experience of transradial coronary intervention for chronic total occlusion. Korean Circ J. 2003. 33:805–812.
13. Tan KH, Sulke N, Taub NA, Watts E, Karani S, Sowton E. Determinants of success of coronary angioplasty in patients with a chronic total occlusion: a multiple logistic regression model to improve selection of patients. Br Heart J. 1993. 70:126–131.
14. Hirokami M, Saito S, Muto H. Anchoring technique to improve guiding catheter support in coronary angioplasty of chronic total occlusions. Catheter Cardiovasc Interv. 2006. 67:366–371.
15. Rathore S, Terashima M, Suzuki T. Value of intravascular ultrasound in the management of coronary chronic total occlusions. Catheter Cardiovasc Interv. 2009. 74:873–878.
16. Singh M, Bell MR, Berger PB, Holmes DR Jr. Utility of bilateral coronary injections during complex coronary angioplasty. J Invasive Cardiol. 1999. 11:70–74.
17. Kawamura A, Jinzaki M, Kuribayashi S. Percutaneous revascularization of chronic total occlusion of left anterior descending artery using contralateral injection via isolated conus artery. J Invasive Cardiol. 2009. 21:E84–E86.
18. Srivatsa S, Holmes D Jr. The histopathology of angiographic chronic total coronary artery occlusions N changes in neovascular pattern and intimal plaque composition associated with progressive occlusion duration. J Invasive Cardiol. 1997. 9:294–301.
19. Carlino M, Latib A, Godino C, Cosgrave J, Colombo A. CTO recanalization by intraocclusion injection of contrast: the microchannel technique. Catheter Cardiovasc Interv. 2008. 71:20–26.
20. Saito S. Waksman R, Saito S, editors. Wire control handling technique. Chronic Total Occlusions. 2009. 1st ed. West Sussex: Wiley-Blackwell;88–90.
21. Saito S, Tanaka S, Hiroe Y, et al. Angioplasty for chronic total occlusion by using tapered-tip guidewires. Catheter Cardiovasc Interv. 2003. 59:305–311.
22. Mitsudo K, Yamashita T, Asakura Y, et al. Recanalization strategy for chronic total occlusions with tapered and stiff-tip guidewire. J Invasive Cardiol. 2008. 20:571–577.
23. Rathore S, Matsuo H, Terashima M, et al. Procedural and in-hospital outcomes after percutaneous coronary intervention for chronic total occlusions of coronary arteries 2002 to 2008: impact of novel guidewire techniques. JACC Cardiovasc Interv. 2009. 2:489–497.
24. Surmely JF, Suzuki T. Waksman R, Saito S, editors. Parallel-wire techniquse. Chronic Total Occlusions. 2009. 1st ed. West Sussex: Wiley-Blackwell;83–85.
25. Matsubara T, Murata A, Kanyama H, Ogino A. IVUS-guided wiring technique: promising approach for the chronic total occlusion. Catheter Cardiovasc Interv. 2004. 61:381–386.
26. Rathore S, Terashima M, Suzuki T. Value of intravascular ultrasound in the management of coronary chronic total occlusions. Catheter Cardiovasc Interv. 2009. 74:873–878.
27. Werner GS, Diedrich J, Schlz KH, Knies A, Kreuzer H. Vessel reconstruction in total coronary occlusions with a long subintimal wire pathway: use of muiltiple stents under guidance of intravascular ultrasound. Cathet Cardiovasc Diagn. 1997. 40:46–51.
28. Rathore S, Katoh O, Matsuo H, et al. Retrograde percutaneous recanalization of chronic total occlusion of the coronary arteries: procedural outcomes and predictors of success in contemporary practice. Circ Cardiovasc Interv. 2009. 2:124–132.
29. Werner GS, Ferrari M, Heinke S, et al. Agiographic assessment of collateral connections in comparison with invasively determined collateral function in chronic coronary occlusions. Circulation. 2003. 107:1972–1977.
30. Surmely JF, Katoh O, Tsuchikane E, Nasu K, Suzuki T. Coronary septal collaterals as an access for the retrograde approach in the percutaneous treatment of coronary chronic total occlusions. Catheter Cardiovasc Interv. 2007. 69:826–832.
31. Kimura M, Katoh O, Tsuchikane E, et al. The efficacy of a bilateral approach for treating lesions with chronic total occlusions the CART (controlled antegrade and retrograde subintimal tracking) registry. JACC Cardiovasc Interv. 2009. 2:1135–1141.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download