Abstract
Endocardial fibroelastosis (EFE) is characterized by deposition of collagen and elastin leading to ventricular hypertrophy and diffuse endocardial thickening. Here we report (for the first time in Korea) the case of a EFE presenting with heart failure. The patient was a 57-year-old woman who had complained of dyspnea on exertion {New York Heart Association (NYHA) functional class 3} and abdominal distension at the time of hospital admission. Echocardiography showed severe diastolic dysfunction with normal systolic function. On MRI, the contrast-enhanced delayed myocardial image demonstrated hyperenhancement in the endocardium. Owing to progressive heart failure, the patient was transplanted. Histological examination of the explanted heart showed irregularly thickened endocardium with fibrosis and elastosis in the both ventricles, compatible with the diagnosis of EFE.
Endocardial fibroelastosis (EFE) is a disorder of fetuses and infants of unclear etiology that is characterized by deposition of collagen and elastin, and that leads to ventricular hypertrophy and diffuse endocardial thickening.1) However, a recent study has shown that even in adult heart recipients, EFE is a relatively frequent finding in histological samples of explanted hearts.2) The basic feature of the disease is the formation of fibrous tissue on the endocardium in the inflow tract of the right or left ventricle or both, leading to partial obliteration of the ventricle with decreased ventricular distensibility and impaired diastolic filling.3)
In Korea there were no reports of a case of EFE in adults. Therefore, we now report the case of a 57-year-old woman with EFE confirmed by histological examination of her explanted heart after heart transplantation.
A 57-year-old female was admitted to our hospital due to heart failure. She had been diagnosed with restrictive cardiomyopathy secondary to endomyocardial fibrosis when she was 38 years old. Afterward, she had remained in good functional status until she was 53-years-old.
When the patient was admitted to our hospital, she complained of dyspnea on exertion {New York Heart Association (NYHA) functional class 3} and abdominal distension over the last several months. The initial blood pressure was 112/56 mmHg, the heart rate 67/minute, the respiratory rate 18/minute, and temperature 35.9℃. On physical examination, increased jugular venous pressure with Kussmaul's sign and decreased breathing sounds in both lower lung fields were observed. In addition, there were a shifting dullness on her distended abdomen and peritibial pitting edema (grade 3).
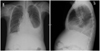
Laboratory findings showed anemia (Hb 7.9 g/dL), hypoalbuminemia (2.0 g/dL), and elevated N-terminal pro-B-type natriuretic peptide level (359.0 pg/mL). The electrocardiogram showed no specific abnormality. On the chest X-ray, bilateral pleural effusion was observed (Fig. 1). The echocardiogram showed severe diastolic dysfunction (grade 4) with normal ventricular size and normal systolic function. Coronary angiography was performed and showed normal coronary arteries. On cardiac catheterization, mild pulmonary hypertension (pulmonary arterial pressure 40/13 mmHg), diastolic equilibrium of four chambers, and dip and plateau configuration of ventricular pressure were observed. On cardiac MRI, a contrast-enhanced delayed myocardial image demonstrated endocardial hyperenhancement in diffuse area of both ventricles (Fig. 2). Endomyocardial biopsy revealed subendocardial widening with fibrosis.
Finally, the patient received a heart transplantation 5 months later because of progressive heart failure. The gross specimen of the explanted heart showed diffuse whitish endocardial thickening (Fig. 3A). There were neither valvular abnormalities nor other malformations. Histological examination showed irregularly thickened endocardium with fibrosis and elastosis in the left ventricle, compatible with the diagnosis of EFE (Fig. 3B).
On the thirtieth day after the operation, she was discharged without any residual complications. She has been regularly followed up for one year. Her one-year follow-up echocardiography and cardiac biopsy showed normal cardiac function and no allograft rejection.
EFE usually occurs in infants and young children who present with signs of congestive heart failure. EFE is an adverse prognostic factor for children and adolescents with dilated cardiomyopathy.4) There have been many reports about EFE presenting in fetuses, infants and children including a Korean report, in which EFE was proved by autopsy after sudden unexpected death of the infant.5-7) There was a Korean paper about idiopathic restrictive cardiomyopathy in children, but the pathologic diagnosis was not investigated.8) Several cases with EFE in young adults have also been reported in other countries.4)9) Interestingly, however, our patient showed an unusually benign course despite extensive fibroelastosis and remained in good functional status until her 50s.
The etiology of EFE is unknown. It may be secondary to congenital heart disease, mainly left ventricle obstructive malformations and hypoplastic left ventricle. As for primary EFE, different pathogenetic mechanisms have been suggested, such as genetic factors, viral infections, carnitine deficiency, or transplacental crossing of maternal antibodies (anti-Ro, anti-La). It has also been suggested to be a nonspecific response to chronic myocardial dysfunction.4) In our case, we could not find any etiology for the EFE.
The diagnosis of EFE is difficult to establish because clinical symptoms, electrocardiographic findings and even echocardiographic findings are nonspecific.10) There have been papers that concluded that cardiac MRI and CT could be useful in establishing the presence of EFE.10-12) Stranzinger et al.10) reported that, on MRI, EFE manifested at the endocardial surface as a rim of hypointense signal in the perfusion sequences and as a rim of hyperintense signal in the myocardial delayed-enhancement sequences. On MRI for our case, we also noticed a hyperintense signal along the endocardium in the contrast-enhanced delayed myocardial image. The MRI image was obtained using a balanced turbofield echo technique after injection of gadobutrol. The diagnosis of EFE was confirmed after cardiac transplantation. As the gross specimens of other reported cases showed,4)13) we could see the typical EFE finding referred to as a whitish fibrous tissue lining the endocardium.14)
In summary, we report, for the first time in Korea, the case of a 57-year-old woman with primary EFE presenting as heart failure. We observed very similar findings in her MRI image and in the examination of her explanted heart with previous cases of EFE reported in other countries.
Figures and Tables
Fig. 2
On cardiac MRI, the contrast-enhanced delayed myocardial image demonstrated endocardial hyperenhancement (arrows) in diffuse areas of both ventricles. A: short-axis view. B: four-chamber view.
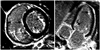
Fig. 3
Gross and microscopic examination of the explanted heart. A: explanted heart specimen, cut along a basal ventricular short-axis plane, showing significant whitish endocardial thickening in both ventricles. B: a low-power photomicrograph showing areas of interstitial fibroelastosis (arrows) (Hematoxylin and Eosin staining ×100).
References
1. Pedra SR, Smallhorn JF, Ryan G, et al. Fetal cardiomyopathies: pathogenic mechanisms, hemodynamic findings, and clinical outcome. Circulation. 2002. 106:585–591.
2. de Leeuw N, Ruiter DJ, Balk AH, de Jonge N, Melchers WJ, Galama JM. Histopathologic findings in explanted heart tissue from patients with end-stage idiopathic dilated cardiomyopathy. Transpl Int. 2001. 14:299–306.
3. Schneider U, Jenni R, Turina J, Turina M, Hess OM. Long-term follow up of patients with endomyocardial fibrosis: effects of surgery. Heart. 1998. 79:362–367.
4. Perez David E, Prieto Arevalo R, Fernandez-Aviles F. Endocardial fibroelastosis in dilated cardiomyopathy in a 28-year-old transplant recipient. Eur Heart J. 2009. 30:477.
5. Takahashi S, Kanetake J, Moriya T, Funayama M. Sudden infant death from dilated cardiomyopathy with endocardial fibroelastosis. Leg Med (Tokyo). 2008. 10:277–280.
6. Moon YO, Choi HK, Her JA, et al. Sudden unexpected death in infancy: analysis of 34 cases including 13 autopsies. J Korean Pediatr Soc. 2002. 45:1065–1074.
7. Chou YT, Wang JK, Chou HC. Primary endocardial fibroelastosis with dilated cardiomyopathy: report of one case. Acta Paediatr Taiwan. 2007. 48:213–216.
8. Bae EJ, Cheon EJ, Yun YS. Clinical profile and outcome of idiopathic restrictive cardiomyopathy in children. Korean Circ J. 2001. 31:427–433.
9. Fuchs U, Zittermann A, Schulz U, et al. Unusual case of an 18-year-old heart transplant recipient with endocardial fibroelastosis. Transplant Proc. 2006. 38:1511–1513.
10. Stranzinger E, Ensing GJ, Hernandez RJ. MR findings of endocardial fibroelastosis in children. Pediatr Radiol. 2008. 38:292–296.
11. Maredia N, English K, Greenwood J. Assessment of endocardial fibroelastosis by cardiac MRI. Can J Cardiol. 2008. 24:e33.
12. Suh SY, Kim EJ, Yong HS, et al. Endocardial fibroelastosis demonstrated on multidetector computed tomography. Int J Cardiol. 2008. 124:e51–e52.
13. Corradi D, Tchana B, Miller D, et al. Dilated form of endocardial fibroelastosis as a result of deficiency in respiratory-chain complexes I and IV. Circulation. 2009. 120:e38–e40.
14. Stehbens WE, Delahunt B, Zuccollo JM. The histopathology of endocardial sclerosis. Cardiovasc Pathol. 2000. 9:161–173.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download