Abstract
We successfully rescued a patient whose coronary artery perforated following implantation of a drug-eluting stent (DES), by deploying a stent-graft in symptomatic myocardial bridging. Our case demonstrated that coronary perforation could be handled without difficulty when perforated myocardial bridging is confined to the interventricular groove
Myocardial bridging is defined as an epicardial coronary artery that goes intramurally through the myocardium beneath the muscle bridge. While generally benign, myocardial bridges can cause ischemia, ventricular tachyarrhythmias, atrioventricular block, and sudden cardiac death.1)2) For symptomatic patients, various therapeutic approaches have been attempted, but the optimal treatment of myocardial bridging still remains controversial.3)4) Coronary stenting has been another therapeutic option with medical and surgical treatment, but the high risk of perforation and high rate of in-stent restenosis have limited its use.5-10) Recently, we experienced a patient with a perforated coronary artery after implantation of a drug-eluting stent (DES) that was successfully rescued by deployment of covered stent in symptomatic myocardial bridging.
A 46 year-old woman who had no coronary risk factors, presented with exertional chest pain for several weeks. The chest pain was typical for angina pectoris and depressed ST segments were noted at the exercise test. Echocardiography revealed normal left ventricular (LV) systolic function {ejection fraction (EF)=72%} without any regional wall motion abnormality. We performed coronary angiography which showed significant stenosis (up to 80%) aggravated by severe myocardial bridging at the mid-portion of the left anterior descending (LAD) artery (Fig. 1A) without significant stenotic lesions at other coronary arteries. The pain was not relieved by optimal medical treatment for 2 weeks. So we decided to do a percutaneous coronary intervention (PCI) at the LAD lesion. Through a 7 Fr Judkins guiding catheter, the lesion was easily crossed with a 0.014 Choice floppy guidewire. Predilatation was performed with a maverick balloon catheter (2.5×15 mm, Boston Scientific, Natick, MA, USA) at 10 atmospheres for 20 seconds. We deployed a Taxus stent (3.5×16 mm, Boston Scientific, Natick, MA, USA) according to the size of the predilated balloon catheter. The stent was inflated up to a nominal pressure 8 atmospheres. But the middle segment of the lesion was not compliant, so the stent was not fully expanded with nominal pressure. We gradually inflated the stent using 16 atmospheres (Fig. 1B). In the mean time, the coronary artery was perforated and some extravasation of contrast media was observed in the pericardium (Fig. 1C). But, the patient's vital signs were stable (blood pressure 115/70 mmHg) with only a mild increase in heart rate. Contrast media extravasation still persisted on follow-up angiography. However, because the perforated site was entrapped intramurally through the myocardium in the interventricular groove, there was no evidence of accumulated blood at the dependent position of the pericardium on fluoroscopy or echocardiography. So we decided to observe the patient with close monitoring of symptoms and vital signs. After 10 minutes, angiography revealed continued contrast media extravasation from the perforated site. But echocardiography showed no evidence of pericardial effusion. On an intravascular ultrasound (IVUS) study, a large perforated site and a perivascular hematoma were observed in the mid-portion of the deployed stent (Fig. 1C). Based on the IVUS findings, a Jo covered stent (3×19 mm, Jomed International AB, Helsingborg, Sweden) was deployed at the perforation site. Contrast media extravasation was not noted after deployment of the covered stent (Fig. 1E). Follow-up echocardiography showed a minimal amount of pericardial effusion without hemodynamic instability (Fig. 1F). Several days after the procedure, the patient was discharged. Four months after the procedure, CT angiography showed no evidence of residual hematoma or pseudoaneurysm (Fig. 2A and B). Follow-up coronary angiography performed at 8 months after the procedure showed good distal flow with minimal stenosis at the proximal edge of the stent (Fig. 2C). The patient is doing well without exertional chest pains.
Although coronary stenting is an effective interventional approach to improve symptoms in selected patients with myocardial bridging, it is associated with a high risk of coronary perforation.5-7) The reason for this phenomenon is not clear. Autopsy findings showed that tunneled segments in myocardial bridging tend to be deficient in vascular smooth muscle density, which may be more prone to vascular disruption during high inflation pressures during PCI.11)12) Another study revealed that the vessel area in the myocardial bridge segment was significantly smaller than that in the adjacent reference segments proximal and distal to the myocardial bridge throughout the cardiac cycle.13) This finding might explain the higher rate of coronary perforation associated with coronary stent implantation for myocardial bridges. The histological and anatomical differences in tunneled coronary arteries may require an adjustment in stent diameter and inflation pressures to help reduce the risk of coronary perforation. In our case, we selected an oversized stent, resulting in coronary perforation. Pre-interventional IVUS may be helpful in selection of an appropriate size of stent and, in particular, in cases that require high inflation pressures for optimal stent implantation. Although coronary perforation is an uncommon complication following PCI, it usually causes a catastrophic result including cardiac tamponade, emergency coronary artery bypass surgery, or pseudoaneurysm formation, with the potential for late coronary rupture and death.14)15) But, in myocardial bridging as observed in our patient, because the perforation site and extravasated blood are mainly confined in the interventricular groove, perforation itself usually does not cause hemodynamic instability. We were able to evaluate the lesion using IVUS to decide on implantation of a covered stent after perforation.
Coronary stenting in myocardial bridging has been associated with a high restenosis rate.9) Possible factors for this include shear stress from persistent external compression from myocardial bridges, causing neointimal proliferation, and long stent and recoil phenomena when inadequate pressures are used for stent deployment. A DES was chosen as a feasible alternative to surgery because it had the ability to reduce restenosis. In our case, we performed PCI with a DES, which also showed good distal flow with minimal restenosis at the proximal edge of the stent at follow-up angiography.
Our case showed that, even though coronary perforation had occurred in myocardial bridging, the perforation site was confined in the interventricular groove. Therefore, it could be managed more easily than conventional coronary perforation.
Figures and Tables
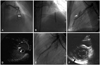 | Fig. 1A: angiography revealed 80% stenosis aggravated by severe muscular bridging at the mid LAD. B: at a nominal pressure of, the stent was not fully expanded in the middle segment of the lesion. So, we inflated the stent with a high pressure, up to 16 atm. C: on follow-up angiography, the coronary artery was perforated and extravasation of contrast into the pericardium was noted at the site of the lesion. D: an IVUS study showed the perforation site and a perivascular hematoma along the lesion. E: after implantation of a covered stent, leaking flow from the coronary lesion was no longer noted. F: follow-up echocardiography showed a minimal amount of pericardial effusion (arrow) without hemodynamic instability. LAD: left anterior descending, IVUS: intravascular ultrasound. |
 | Fig. 2A and B: four months after the stent procedure, CT angiography showed no evidence of residual hematoma or pseudoaneurysm (arrow). C: follow-up coronary angiography performed at 8 months after the procedure showed good distal flow with minimal stenosis at the proximal edge of the stent (arrow). |
References
1. Tauth J, Sullebarger T. Myocardial infarction associated with myocardial bridging: case history and review of the literature. Cathet Cardiovasc Diagn. 1997. 40:364–367.
2. Cutler D, Wallace JM. Myocardial bridging in a young patient with sudden death. Clin Cardiol. 1997. 20:581–583.
3. Alegria JR, Herrmann J, Holmes DR Jr, Lerman A, Rihal CS. Myocardial bridging. Eur Heart J. 2005. 26:1159–1168.
4. Mohlenkamp S, Hort W, Ge J, Erbel R. Update on myocardial bridging. Circulation. 2002. 106:2616–2622.
5. Broderick TM, Kereiakes DJ, Whang DD, Toltzis RJ, Abbottsmith CW. Myocardial bridging may predispose to coronary perforation during rotational atherectomy. J Invasive Cardiol. 1996. 8:161–163.
6. Hering D, Horstkotte D, Schwimmbeck P, Piper C, Bilger J, Schultheiss HP. Acute myocardial infarct caused by a muscle bridge of the anterior interventricular ramus: complicated course with vascular perforation after stent implantation. Z Kardiol. 1997. 86:630–638.
7. Berry JF, von Mering GO, Schmalfuss C, Hill JA, Kerensky RA. Systolic compression of the left anterior descending coronary artery: a case series, review of the literature, and therapeutic options including stenting. Catheter Cardiovasc Interv. 2002. 56:58–63.
8. Kim BJ, Gwon HC, Hong JS, et al. Clinical aspects of coronary artery perforation during percutaneous coronary intervention. Korean Circ J. 2003. 33:277–283.
9. Haager PK, Schwartz ER, vom Dahl J, Klues HG, Reffelmann T, Hanrath P. Long term angiographic and clinical follow up in patients with stent implantation for symptomatic myocardial bridging. Heart. 2000. 84:403–408.
10. Choi SH, Shim SJ, Byun KH, Choi D, Shim WH. A case of coronary stenting in the management of myocardial ischemia caused by myocardial bridging. Korean Circ J. 2001. 31:940–944.
11. Morales AR, Romanelli R, Tate LG, Boucek RJ, de Marchena E. Intramural left anterior descending coronary artery: significance of the depth of the muscular tunnel. Hum Pathol. 1993. 24:693–701.
12. Glagov S, Zarins C, Giddens DP, Ku DN. Hemodynamics and atherosclerosis: insights and perspectives gained from studies of human arteries. Arch Pathol Lab Med. 1988. 112:1018–1031.
13. Qian J, Zhang F, Wu H, et al. Size of coronary artery in myocardial bridge compared with adjacent nontunneled left anterior descending coronary artery. Am J Cardiol. 2007. 99:1653–1655.
14. Ajluni SC, Glazier S, Blankenship L, O'Neill WW, Safian RD. Perforations after percutaneous coronary interventions: clinical, angiographic, and therapeutic observations. Cathet Cardiovasc Diagn. 1994. 32:206–212.
15. Gruberg L, Pinnow E, Flood R, et al. Incidence, management and outcome of coronary artery perforation during percutaneous coronary intervention. Am J Cardiol. 2000. 86:680–682.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download