Abstract
In the last decade the hybrid procedure has emerged as an alternative stage I palliation in neonates with hypoplastic left heart syndrome (HLHS). This review discusses the historical aspect, surgical and interventional techniques, current outcomes and future direction of this procedure. Hybrid palliation yields equivalent but not superior stage I palliation survival and comparable 1-year survival to conventional Norwood palliation, comparable prestage II hemodynamics and pulmonary artery growth, and preserved ventricular function in stage II palliation. Hybrid palliation utilizes significantly less resource and shortens postoperative recovery. In comprehensive stage II palliation the impact of pulmonary artery reconstruction on subsequent pulmonary artery growth has not been determined and should be further investigated. A prospective, randomized trial is warranted to compare these two surgical strategies for neonates with hypoplastic left heart syndrome.
Neonates with hypoplastic left heart syndrome (HLHS) and its variants have diminutive left-sided structures with systemic outflow obstruction and subsequent duct-dependent systemic, cerebral, and coronary circulations.1) These anatomic features result in an inherently unstable physiology, in which the systemic circulation can be critically compromised by minor changes in resistances of the systemic and/or pulmonary vasculature. The essential aims of stage I palliation in the neonatal period are to establish an unobstructed systemic outflow, unobstructed intra-atrial communication, and effective pulmonary blood source. The Norwood procedure has been the sole option to achieve these purposes for nearly 20 years.2)3) Despite a significant improvement in survival after stage I palliation, early mortality remains as high as 20-30%4)5) with significant morbidity, including neurologic injury.6)7) The high mortality and morbidity stems from the following two components: an essentially unstable 'in-parallel' circulation of Norwood physiology with a systemic-to-pulmonary shunt, and surgical stress driven by cardiopulmonary bypass (CPB), deep hypothermic circulatory arrest (DHCA), and the subsequent systemic inflammatory response.
The hybrid procedure {placement of bilateral pulmonary artery (PA) bands and a ductal stent} has emerged in the last decade as an alternative stage I palliation for neonates with HLHS.8-10) The potential but unproven advantages of this strategy include avoiding the use of CPB, cardioplegic cardiac arrest, and DHCA, which minimizes the extent of surgery, including the degree of the inflammatory response during stage I palliation, thereby improving clinical outcomes. However, there are potential disadvantages of the hybrid procedure, including the unrepaired hypoplastic aortic arch, which carries the risk of malperfusion due to retrograde aortic arch obstruction, possible restriction of an intraatrial communication requiring re-intervention, mechanical distortion of the branch PAs, and significantly greater surgical requirements during comprehensive stage II palliation. The initial experiences reported high morbidity and mortality mainly due to the learning curve of an emerging strategy.8)9)11) To date, no significant survival benefit of the hybrid approach over the conventional Norwood approach has been reported. Herein we review the history, surgical and interventional techniques, current outcomes, and future direction of the hybrid strategy for patients with HLHS.
Bilateral PA banding was described by Dr. Norwood based on his early experience of surgical palliation for HLHS before he established the 'Norwood procedure'12) and has been an option to stabilize critically ill neonates with HLHS.13) This procedure, however, did not become a hybrid procedure until ductal stenting became clinically available.
In the past, maintenance of arterial duct patency in patients with duct-dependent pulmonary or systemic circulation has only been secured with prostaglandin infusion. Extensive animal studies in the early 1990s showed the feasibility of stent implantation in the arterial duct to maintain chronic patency.14) Gibbs et al.15) first reported a combined surgical and interventional approach as an alternative to the conventional stage I palliation for HLHS in 1993, which consisted of bilateral PA banding, surgical or interventional creation of an unobstructed intraatrial communication, and stenting of an arterial duct. Two of four patients (50%) died within 2 weeks, both of which were related to right ventricular failure. The arterial ducts were both patent, although one had some distal ductal restriction because the stent did not cover the entire duct. The nature of right ventricular failure was not determined. Their subsequent experience reported in 1999 essentially showed similar results: 5 of 8 patients died due to right ventricular failure (4 patients died within 3 months, and 1 patient died at 30 months; 16).
They recommended against performing ductal stenting. In 2002, Akintuerk and colleagues10) reported the first successful series involving a 'hybrid' approach for HLHS in which 9 of 11 patients (81%) were successfully palliated with ductal stenting and bilateral PA banding, and subsequently underwent either reconstructive surgery or heart transplantation. By the mid-2000s, several institutes, including our institute, initiated hybrid programs as an alternative to the conventional Norwood program.
Neonatal palliation without the use of CPB in patients with HLHS has been independently developed in pediatric heart transplantation programs. In 1993, Ruiz et al.17) reported their experience of ductal stenting as a bridge to transplantation. Four of 5 patients (80%) successfully underwent heart transplantation after ductal stenting. More recently, Mitchell et al.18) reported the efficacy of bilateral PA banding as a bridge to heart transplantation in order to prevent irreversible pulmonary vascular disease.
There is no consensus or guidelines on hybrid procedures in patients with HLHS. Every institute has independently developed the indications for a hybrid procedure and patient selection. In 2006, Bacha et al.11) reported their initial experience on hybrid stage I procedures in high-risk neonates. They defined 'high-risk' as the presence of aortic atresia, severe non-cardiac anomalies, low body weight (<2.5 kg), an intact or highly restrictive atrial septum, prematurity, and poor ventricular function. Galantowicz et al.,9)19) who pioneered and popularized the hybrid approach, and intended to treat all neonates with HLHS with a hybrid approach, including patients with aortic atresia and/or a diminutive aortic arch.
In The Hospital for Sick Children, our indications for the hybrid procedure are all neonates with HLHS, including aortic atresia, diminutive aortic arch and/or stenotic aortic isthmus. The HLHS variants, such as tricuspid atresia with transposed great arteries, unbalanced atrioventricular septal defect with aortic arch obstruction, or double inlet left ventricle with transposed great arteries, are all considered an indication for the hybrid procedure. The decision-making process is unique in our institute. The type of surgical palliation is typically determined through interdisciplinary conferences on a case-by-case basis. Staged surgical palliation with the standard Norwood approach, hybrid approach, and primary transplantation are equally offered to the patient's family by the cardiologist, and no specific decision-making protocol is applied. Some patients who are considered at high-risk candidate for the Norwood procedure, i.e., neonates with intracranial hemorrhage or extreme prematurity and/or low body weight, tend to be treated with the hybrid approach.
In patients with aortic atresia, the coronary and cerebral circulations are entirely dependent on retrograde blood flow through the aortic isthmus. If there are any signs of pre-operative obstruction at the aortic isthmus or retrograde aortic arch, deployment of a ductal stent can result in acute or chronic obstruction of the aortic isthmus, leading to coronary and cerebral ischemia.20) This specific anatomic feature can be a relative contraindication for a 'conventional' hybrid procedure21) unless specific measures are applied to secure the coronary and cerebral circulations. The following strategies have been proposed: 1) prophylactic main PA to innominate artery shunt during stage I palliation {i.e., a 'reverse Blalock-Taussig (BT) shunt'},20) 2) stent placement in the stenotic aortic isthmus, and 3) avoidance of the hybrid procedure in favor of the Norwood procedure or transplantation.
Hybrid stage I palliation has been previously described.9)10) Herein, the current techniques used in The Hospital for Sick Children are described. The procedure is performed in the catheterization laboratory, which is referred to as the 'hybrid suite'. Under general anesthesia, a median sternotomy is made and the thymus is resected. Bilateral PA banding is achieved using a 3.5 mm polytetrafluoroethylene graft, which is divided longitudinally and wrapped around the branch PAs for a width of approximately 3 mm.
The bands are then secured to the adventitia of the main PA with 5-0 or 6-0 non-absorbable polypropylene sutures to avoid distal dislodging. A vascular clip is placed at the proximal edge of the left PA band to guide the interventionist for ductal stenting.
The patient is partially heparinized, a purse-string suture is placed on the main PA, and a 6-Fr sheath is inserted. The ductal stent is deployed through the sheath under fluoroscopic guidance, achieving stage I palliation (Figs. 1 and 2). We currently use a self-expanding stent, 20 mm in length and 8-10 mm in diameter (eV3 Protégé; ev3 Endovascular, Inc. Peripheral Vascular, Plymouth, MN, USA).
Milrinone is commonly used in post-operative care to optimize cardiac output and provide systemic vasodilatation. This management strategy stems from our early observation that patients who underwent a hybrid procedure had diminished cardiac output, high systemic vascular resistance, and a high pulmonary-to-systemic flow ratio (Qp/Qs) despite avoiding CPB/DHCA (see Early post-operative physiology)22)
In our early experience, atrial septectomies were typically performed as part of the same procedure; currently atrial septectomies and stenting are deferred until the intra-atrial communication becomes restrictive.
In patients with aortic atresia or those thought to have severely restricted prograde aortic flow, a revered BT shunt is prophylactically placed from the main PA to the innominate artery.20) After bilateral PA banding is achieved, a side-biting clamp is placed on the main PA and proximal anastomosis is made using a 3.5 or 4 mm polytetrafluoroethylene graft on the anterior wall of the main PA. Distal anastomosis is then performed to the proximal innominate artery with a standard anastomotic technique. The flow in the shunt is directed from the main PA to the innominate artery. Ductal stenting is then performed.
Close monitoring with weekly or biweekly clinic visits and echocardiograms are performed. The arm-leg blood pressure difference is measured at every clinic visit to exclude any signs of aortic arch obstruction. Echocardiographic follow-up is particularly focused on the presence or absence of flow acceleration in the aortic isthmus, indicating progression of retrograde aortic arch obstruction. The status of the atrial septum is also carefully monitored, determining the timing of atrial septectomy. There are no certain criteria for intervention on the atrial septum. The decision is made based on the balance between the patient's clinical status and the pressure gradient across the intra-atrial communication. A mild-to-moderate pressure gradient up to 8-10 mmHg is acceptable as long as the patient is thriving. Prompt intervention is required when the patient has symptoms of left atrial hypertension, such as poor feeding, an increased respiratory rate, and a decrease in arterial saturation. Ventricular function and atrioventricular valve regurgitation are of great importance during the follow-up echocardiographic examination. Depressed ventricular function can be a result of retrograde aortic arch obstruction in the setting of minimal or no antegrade aortic flow, or stenosis of a reverse BT shunt. The patency and flow pattern of the reverse BT shunt is documented. Anti-platelet and/or anti-coagulation is indicated only if the patient has a reverse BT shunt. Anti-platelet therapy is initiated if the patient has a stent across the atrial septum. Pre-stage II cardiac catheterization is electively performed at 3-4 months of age unless intervention for the atrial septum is necessary at an earlier age.
Stage II palliation is performed at 4-6 months of age. After re-sternotomy, CPB is initiated with the arterial cannula placed in the main PA (or on the ascending aorta if it is large enough to be cannulated) and bicaval cannulations. The patient is cooled in preparation for DHCA. The branch PA bands are removed. Cardioplegic cardiac arrest is achieved and atrial septectomy is performed. Under DHCA with regional cerebral perfusion, aortic arch reconstruction is performed using a pulmonary homograft patch. A proximal Damus-Kaye-Stansel anastomosis is achieved with a standard technique. Branch PAs are enlarged with an autologous pericardial patch from hilum-to-hilum in most of the patients. Finally, bidirectional cavopulmonary shunt is achieved with a standard technique in the on-pump beating state, while rewarming the patient. The sternum can be closed in most of the patients.
Recently, we have used retained stented duct tissue to facilitate aortic arch reconstruction.23) After DHCA is initiated, the main PA is transected (Fig. 3A) and the origin of the branch PAs is resected as a single large button (Fig. 3B and C). The area of apposition between the ascending aorta and the stented ductus is divided all the way to the orifice of the aortic isthmus (Fig. 3D). All stent material along the posterior aspect of the proximal descending aorta is resected. The remaining portion of the stented ductus is used as a 'patch' for aortic arch reconstruction. The aortic arch is reconstructed by sewing the lateral edges of the divided ductus to the divided native aortic arch.
The potential advantages of this modification are as follows: 1) the stented material is already in place, as in the natural transverse aortic arch, 2) the continuity between the ductal material and the descending aorta avoids the need for exposure of this area, thereby potentially reducing the risk of bleeding and recurrent laryngeal nerve injury, and 3) avoidance of homograft material may improve the potential for future transplantation by avoiding immunologic sensitization. Potential disadvantages include possible aneurysmal formation and recurrent obstruction at the level of the aortic isthmus.
A representative angiogram of the aortic arch reconstruction using the stented ductus technique is shown in Fig. 4. The posterior aspect of the stented ductus has a fine natural curve similar to the native aortic arch, which increases the amount of space available for the left branch PA.
The Fontan operation is typically performed at 2-3 years of age. The timing of surgery and operative techniques used for the Fontan procedure are similar to conventional approaches. Briefly, the Fontan connection is achieved with a 20-24 mm polytetrafluoroethylene tube using an extracardiac technique. The procedure is performed in the on-pump beating state with aortic and bicaval cannulation. A 4 mm fenestration between the graft and the right-sided atrium is routinely created. An early extubation strategy is applied.
Comparison of the post-stage I physiology between the Norwood and hybrid palliation is of interest within the context of very different modes of surgical techniques. The Norwood palliation involves CPB/DHCA and results in an in-parallel circulation with a BT shunt. On the other hand, hybrid palliation is a less invasive non-CPB procedure and the native 'banded' PAs serves as a pulmonary blood source. We compared the early post-operative physiology between the Norwood (n=13) and hybrid stage I (n=6) using respiratory mass spectrometry.22) In the first 48 hours, hybrid patients have a lower cardiac output, higher systemic vascular resistance, higher pulmonary-to-systemic flow ratio, decreased oxygen delivery and consumption, an increased oxygen extraction ratio (Qp/Qs), and an increased lactate level (Fig. 5).22) Those hemodynamic trends reversed after 48 hours. Therefore, despite the non-invasive nature of hybrid stage I procedures, there are acute alterations in post-operative physiology which can be clinically significant, especially in patients with limited myocardial reserve. After the first 48 hours, this period of diminished oxygen delivery resolves, whereas the Norwood patients have a slower recovery.
There are some important considerations regarding the data. In our institute, early post-operative care for Norwood patients is focused on maximal vasodilation to secure systemic oxygen delivery and afterload reduction on the systemic ventricle.4)24) Therefore, most of the Norwood patients receive milrinone and phenoxybenzamine. In contrast, aggressive systemic vasodilation therapy is not utilized in the hybrid patients. In light of these findings, we currently manage post-operative hybrid stage I patients with a strategy which emphasizes securing cardiac output and systemic oxygen delivery.
Some centers advocate performing hybrid stage I palliation in a staged manner, which entails separate procedures for PA banding and the ductal stenting.25) Schranz et al.25) suggested that staged palliation may result in a reduced physiologic injury. Little data is available to examine this strategy. Currently, we prefer to conduct stage I palliation as a single procedure. We do, however, advocate deferring atrial septostomy until after the patient has recovered from the stage I procedure whenever possible. Maintenance of a small gradient across the atrial septum is well tolerated during the early post-operative period.
Initial reports from different centers showed high mortalities after stage I hybrid palliation, reflecting a steep learning curve.9)11) More recent series, however, have demonstrated that hospital survival after stage I hybrid palliation ranges from 80-97%. It is important to note, however, that some studies excluded high-risk patients from the analysis.10)19)26) Nonetheless, there is little published data making a direct comparison in survival between Norwood and hybrid palliation. We recently published a non-randomized series of Norwood (n=39) and hybrid (n=19) palliation in neonates with HLHS and its variants between 2004 and 2007. This series included all neonates with all anatomic variants since we initiated the hybrid program. There were 4 (21%) operative or interim deaths in the hybrid group and 8 (20.5%) deaths in the Norwood group after stage I palliation (Fig. 6). There was no difference in survival between the patients with or without reverse BT shunts. Four additional Norwood patients underwent heart transplantation after the Norwood procedure. There was one (6.6%) late post-operative death in the hybrid group. The 1-year survival was equivalent between the groups (hybrid 73.7% vs. Norwood 69.2%, p=0.83) (Fig. 7).
We reviewed the pre-stage II hemodynamics and PA growth between thee Norwood and hybrid groups at pre-stage II cardiac catheterization (Table 1). There was no difference in the PA pressure between the groups. There was a non-significant trend toward a lower ventricular end-diastolic pressure and higher mixed venous saturation in the hybrid group compared to the Norwood group (Table 1). There was no difference in Qp/Qs and arterial saturation between the groups. There were non-significant trends toward a larger Nakata index and a lower lobe index in the hybrid group compared to the Norwood group. Both groups had equivalent hemodynamics and PA growth at the pre-stage II evaluation. We have not analyzed the pre-Fontan data in these groups. The effect of a newly reconstructed aortic arch at the comprehensive stage II palliation on PA growth in the hybrid group is uncertain and will be evaluated.
Progressive systemic ventricular dysfunction is an important cause of failure in staged single ventricle palliation for patients with HLHS and is a major indication for heart transplantation. The mechanism of progressive right ventricular failure in HLHS after the Norwood procedure is not entirely clear, but may be related to the following: 1) myocardial damage during stage I of the Norwood procedure with cardioplegic cardiac arrest and DHCA, 2) persistent volume overload due to an inadequately controlled systemic-pulmonary shunt, and 3) relatively high afterload on the systemic right ventricle. In our study, all 15 patients who had prestage II palliation had normal ventricular function and 1 patient (6.6%) had significant atrioventricular valve insufficiency requiring valve repair during stage II palliation. On the other hand, in the Norwood group, 7 patients (26%) had mild ventricular dysfunction and 2 patients (7.4%) had severe ventricular dysfunction and were listed for transplantation. Four patients (15%) in the Norwood group had more than moderate atrioventricular valve insufficiency, of which two underwent valve repair.
It is difficult to reach a strong conclusion regarding the impact of the type of palliation and ventricular function based on our preliminary analysis. Hybrid palliation avoids neonatal exposure to cardioplegic cardiac arrest, but does not prevent some degree of volume and pressure overload to the systemic ventricle. A concern unique to hybrid palliation is that because the systemic outflow obstruction remains untreated at stage I palliation, the coronary circulation can be compromised with retrograde aortic arch obstruction. Progressive narrowing of the aortic isthmus may result in myocardial ischemia and subsequent global ventricular dysfunction.
We recently observed two patients who underwent hybrid stage I and had progressive ventricular dysfunction during the interim stage. One patient had obstruction of the aortic isthmus, diminished aortic arch perfusion, and myocardial dysfunction with minor narrowing of the reverse BT shunt. Ventricular function was improved by the Norwood conversion. Another patient had primary systemic right ventricular failure with an unknown origin requiring heart transplantation after stage II palliation. In summary, systemic ventricle in HLHS is at risk of myocardial injury regardless of the type of palliation. Hybrid patients appear to have better preserved ventricular function at the pre-stage II evaluation, but constant surveillance is important.
DHCA at the time of the Norwood procedure negatively affects neurologic development in patients with HLHS.27)28) One of the potential advantages of hybrid palliation is to avoid neonatal exposure to DHCA, thereby improving long-term neurologic outcomes. There are no data available which compare the hybrid and Norwood strategies with a focus on neurologic development. In hybrid palliation, even though DHCA can be avoided in the neonatal period, the patients are eventually exposed to DHCA at 5-6 months of age. Thus, age at the time of DHCA can be tested to identify if there is an optimum age to undergo arch reconstruction. It is important to recognize that a significant proportion of the neonates with HLHS have pre-operative brain abnormalities,29)30) which may be an important covariate in any analysis of neurologic outcomes comparing the hybrid and Norwood strategies.
Although survival is the most important measure of a palliative strategy, an important secondary parameter is hospital resource utilization. In our experience, there is a shorter combined (stage I and stage II) intubation time, length of intensive care unit stay, and hospital stay in the hybrid group compared to the Norwood group.26) Galantowicz et al.19) showed that the vast majority (85% at stage I and II) of the patients were extubated in the first 24 hours, indicating much quicker post-operative recovery in hybrid patients compared to conventional Norwood patients.
We also found that there are time-related trends towards ongoing improvement in intubation time, intensive care unit stay, and hospital stay for the hybrid stage II procedure, indicating that our hybrid palliation strategy is still on a learning curve with further benefits anticipated as we refine our techniques.26) These same parameters have been unchanged from 2004 to 2007 in our Norwood palliation experience, suggesting that our Norwood palliation strategy is less likely to improve in comparison to the hybrid strategy.
Hybrid palliation for HLHS has dramatically evolved in the last 5-10 years. The currently available data suggest that hybrid palliation offers the following: 1) equivalent, but not superior survival at stage I palliation and subsequently comparable 1-year survival to the conventional Norwood palliation, 2) slightly higher, but acceptable hospital mortality at stage II palliation, 3) comparable pre-stage II hemodynamics and PA growth, 4) preserved ventricular function at stage II palliation, 5) significantly less resource utilization and quicker post-operative recovery, and 6) new innovations (i.e., reverse BT shunt and arch reconstruction with a stented arterial duct), which appear to be effective, but will require long-term evaluation.
Hybrid palliation is an appropriate alternative to the conventional Norwood palliation. Our preliminary experience suggests that a prospective randomized trial is warranted to compare the two palliation strategies in institutions which have extensive experience with both strategies. An important endpoint in any comparison will be the impact of surgical palliation on neurologic development.
Figures and Tables
Fig. 1
Angiographic sequence of stent deployment in hybrid stage I palliation. A: the lateral view showing the sheath and guide wire inserted via the main PA through the arterial duct. B: pre-stenting angiogram showing unobstructed aortic isthmus and reasonable retrograde filling of the aortic arch. C: stent deployment. D: post-stenting angiogram showing reasonable retrograde aortic arch blood flow.
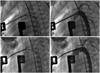
Fig. 2
A: anterior-posterior view showing post-hybrid configuration. The asterisks represent the site of branch PA bands. B: the black asterisk showing a guiding vascular clip placed on the proximal edge of the left PA band. PA: pulmonary artery.

Fig. 3
Modified aortic arch reconstruction in comprehensive stage II palliation using a retained stented duct (reproduced with publisher's permission). A: main PA division. B and C: resection of the branch PAs as a single button. The single arrow showing the aortic isthmus. D: division of apposition between the ascending aorta and the stented duct. E: reconstruction of the aortic arch by sewing the lateral edges of aortic arch and stented duct. F: reconstructed aortic arch. PA: pulmonary artery.
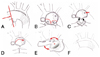
Fig. 4
The lateral view of aortic arch reconstruction using a retained stented duct. The asterisk showing the left branch PA. PA: pulmonary artery.
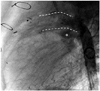
Fig. 5
Hemodynamics and oxygen transport parameters after hybrid (n=6) or Norwood procedures (n=13). SAP: systolic arterial pressure, DAP: diastolic blood pressure, MAP: mean arterial pressure, tPVR: total pulmonary vascular resistance, SVR: systemic vascular resistance, Qp/Qs: pulmonary-to-systemic flow ratio, CO: cardiac output, Qp: pulmonary blood flow, Qs: systemic blood flow, DO2: oxygen delivery; VO2: oxygen consumption, ERO2: oxygen extraction ratio (reproduced with publisher's permission).

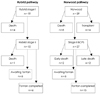
Fig. 6
Clinical outcomes of the patients undergoing hybrid (n=19) or Norwood (n=39) single ventricle palliation. BCPS: bidirectional cavopulmonary shunt.
References
1. Noonan JA, Nadas AS. The hypoplastic left heart syndrome: an analysis of 101 cases. Pediatr Clin North Am. 1958. 5:1029–1056.
2. Norwood WI, Lang P, Hansen DD. Physiologic repair of aortic atresia-hypoplastic left heart syndrome. N Engl J Med. 1983. 308:23–26.
3. Mahle WT, Spray TL, Wernovsky G, Gaynor JW, Clark BJ 3rd. Survival after reconstructive surgery for hypoplastic left heart syndrome: a 15-year experience from a single institution. Circulation. 2000. 102:19 Suppl 3. III136–III141.
4. De Oliveira NC, Ashburn DA, Khalid F, et al. Prevention of early sudden circulatory collapse after the Norwood operation. Circulation. 2004. 110:11 Suppl 1. II133–II138.
5. Stasik CN, Gelehrter S, Goldberg CS, Bove EL, Devaney EJ, Ohye RG. Current outcomes and risk factors for the Norwood procedure. J Thorac Cardiovasc Surg. 2006. 131:412–417.
6. Goldberg CS, Schwartz EM, Brunberg JA, et al. Neurodevelopmental outcome of patients after the fontan operation: a comparison between children with hypoplastic left heart syndrome and other functional single ventricle lesions. J Pediatr. 2000. 137:646–652.
7. Galli KK, Zimmerman RA, Jarvik GP, et al. Periventricular leukomalacia is common after neonatal cardiac surgery. J Thorac Cardiovasc Surg. 2004. 127:692–704.
8. Caldarone CA, Benson L, Holtby H, Li J, Redington AN, Van Arsdell GS. Initial experience with hybrid palliation for neonates with single-ventricle physiology. Ann Thorac Surg. 2007. 84:1294–1300.
9. Galantowicz M, Cheatham JP. Lessons learned from the development of a new hybrid strategy for the management of hypoplastic left heart syndrome. Pediatr Cardiol. 2005. 26:190–199.
10. Akintuerk H, Michel-Behnke I, Valeske K, et al. Stenting of the arterial duct and banding of the pulmonary arteries: basis for combined Norwood stage I and II repair in hypoplastic left heart. Circulation. 2002. 105:1099–1103.
11. Bacha EA, Daves S, Hardin J, et al. Single-ventricle palliation for high-risk neonates: the emergence of an alternative hybrid stage I strategy. J Thorac Cardiovasc Surg. 2006. 131:163–171. e2
12. Lang P, Norwood WI. Hemodynamic assessment after palliative surgery for hypoplastic left heart syndrome. Circulation. 1983. 68:104–108.
13. Pizarro C, Norwood WI. Pulmonary artery banding before Norwood procedure. Ann Thorac Surg. 2003. 75:1008–1010.
14. Coe JY, Olley PM. A novel method to maintain ductus arteriosus patency. J Am Coll Cardiol. 1991. 18:837–841.
15. Gibbs JL, Wren C, Watterson KG, Hunter S, Hamilton JR. Stenting of the arterial duct combined with banding of the pulmonary arteries and atrial septectomy or septostomy: a new approach to palliation for the hypoplastic left heart syndrome. Br Heart J. 1993. 69:551–555.
16. Gibbs JL, Uzun O, Blackburn ME, Wren C, Hamilton JR, Watterson KG. Fate of the stented arterial duct. Circulation. 1999. 99:2621–2625.
17. Ruiz CE, Gamra H, Zhang HP, Garcia EJ, Boucek MM. Brief report: stenting of the ductus arteriosus as a bridge to cardiac transplantation in infants with the hypoplastic left-heart syndrome. N Engl J Med. 1993. 328:1605–1608.
18. Mitchell MB, Campbell DN, Boucek MM, et al. Mechanical limitation of pulmonary blood flow facilitates heart transplantation in older infants with hypoplastic left heart syndrome. Eur J Cardiothorac Surg. 2003. 23:735–742.
19. Galantowicz M, Cheatham JP, Phillips A, et al. Hybrid approach for hypoplastic left heart syndrome: intermediate results after the learning curve. Ann Thorac Surg. 2008. 85:2063–2070. discussion 2070-1.
20. Caldarone CA, Benson LN, Holtby H, Van Arsdell GS. Main pulmonary artery to innominate artery shunt during hybrid palliation of hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 2005. 130:e1–e2.
21. Stoica S, Philips A, Egan M, et al. The Retrograde Aortic Arch in the Hybrid Approach to Hypoplastic Left Heart Syndrome Society for Thoracic Surgeons 45th Annual Meeting. 2009. San Fransicsio.
22. Li J, Zhang G, Benson L, et al. Comparison of the profiles of postoperative systemic hemodynamics and oxygen transport in neonates after the hybrid or the Norwood procedure: a pilot study. Circulation. 2007. 116:11 Suppl. I179–I187.
23. Caldarone CA, Honjo O, Benson LN, Van Arsdell GS. Modification of stage II procedure after hybrid palliation (bilateral pulmonary artery banding and ductal stenting) for hypoplastic left-sided heart syndrome: modified arch reconstruction with retained stented ductus patch. J Thorac Cardiovasc Surg. 2007. 134:1588–1589.
24. Li J, Zhang G, McCrindle BW, et al. Profiles of hemodynamics and oxygen transport derived by using continuous measured oxygen consumption after the Norwood procedure. J Thorac Cardiovasc Surg. 2007. 133:441–448.
25. Schranz D, Michel-Behnke I, Akintuerk H. Letter by Schranz et al regarding article, "Comparison of the profiles of postoperative systemic hemodynamics and oxygen transport in neonates after the hybrid or the Norwood procedure: a pilot study". Circulation. 2008. 117:e296. author reply e297-8.
26. Honjo O, Benson LN, Mewhort HE, et al. Clinical outcomes, program evolution, and pulmonary artery growth in single ventricle palliation using hybrid and Norwood palliative strategies. Ann Thorac Surg. 2009. 87:1885–1892. discussion 1892-3.
27. Kern JH, Hinton VJ, Nereo NE, Hayes CJ, Gersony WM. Early developmental outcome after the Norwood procedure for hypoplastic left heart syndrome. Pediatrics. 1998. 102:1148–1152.
28. Mahle WT, Visconti KJ, Freier MC, et al. Relationship of surgical approach to neurodevelopmental outcomes in hypoplastic left heart syndrome. Pediatrics. 2006. 117:e90–e97.
29. Licht DJ, Shera DM, Clancy RR, et al. Brain maturation is delayed in infants with complex congenital heart defects. J Thorac Cardiovasc Surg. 2009. 137:529–536. discussion 536-7.
30. Miller SP, McQuillen PS, Hamrick S, et al. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. 2007. 357:1928–1938.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download