Abstract
Acute myocardial infarction and subsequent heart failure are leading causes of death worldwide. Stem cell-based therapies have improved cardiac function in recent clinical trials, but cardiomyocyte regeneration has not been demonstrated in human hearts. Angiogenesis and restoration of cardiac perfusion have been successfully performed using bone marrow derived stem cells and other adult stem cells. Resident cardiac stem cells are known to differentiate into multiple heart cell types, including cardiomyocytes. Furthermore, induced pluripotent stem cells are a focus of research due to the great potential for customized stem cell therapy.
The self-repair and renewal capacity of the human heart is known to be very limited.1) After acute myocardial infarction, the ischemic injury of the heart tissue is usually irreversible despite aggressive medical and revascularization treatment.2) Significant loss of functional myocardium usually progresses to irreversible cardiomyopathy. Hence, acute myocardial infarction and subsequent heart failure remain challenging healthcare problems and leading causes of death worldwide. Stem cells have been continuously challenged to regenerate the injured human heart. Now myocardium has become an attractive target of stem cell therapy with vessels, valves, and even conducting systems in the cardiovascular system.
Although considerable research has been done, there has still been no definite advance in the practical human application of stem cell therapy. In this review, we discuss currently available cardiac progenitor cells and focus on recently identified promising progenitor cells for myocardial repair.
It has long been accepted that cardiac muscle cells cannot divide. In 2001, Beltrami et al.3) first found that mitotic figures are present around the ischemic boundary of acute myocardial infarction. This observation indicated that cardiac muscle cells could undertake cell division and repair ischemic damage spontaneously. Anversa et al.4) showed further evidence of the human heart's regenerative capacity. A male patient, who had been transplated with a heart of a female donor, died due to immune rejection. At his autopsy, many cardiomyocytes and arterioles with Y-chromosomes were observed in the female donor's heart. This implies that the pluripotent cells migrated to the donor heart from the recipient's remnant cardiac tissue or from an extra-cardiac organ.
These findings encouraged reconsideration of traditional beliefs concerning cardiac self-regeneration. It is now accepted that the human heart has some capacity to repair myocardial injury. However, the self-regeneration ability of human heart is not naturally potent to influence clinical outcomes. In addition, it is still not known if the replicating cells are in-situ myocytes or migrant cells from other organs. The recruitment and potentiation of self-regenerative cardiomyocytes remain limiting steps for enhancing regeneration of damaged myocardium.
Stem cells are progenitors of specialized cells that impart function to tissues or organs. Stem cells are pluripotent; they can expand, and they possess the potential to differentiate into other cellular lineages.5) Hence, stem cells have become the most promising means of treatment in regenerative cardiovascular medicine. The best established source for adult stem cells is the bone marrow. Bone marrow cells appear to have the ability to repopulate many non-hematopoietic tissues like myoblasts and endothelial cells.6)
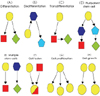
Stem cells are usually classified according to the following conditions: origin (fetal or adult), type of organ or tissue from which the cells are derived, final differentiation fate, and surface cell markers. The cells which are known to have cardiogenic potential, are listed in Table 1. The frequently used terms in stem cell biology are listed and defined in Table 2. The terminology is illustrated to facilitate easy understanding (Fig. 1).
The precise cellular and molecular events that generate pluripotency are largely undetermined. The intra-cellular processes are illustrated in Fig. 2, which shows the several steps that regulate pluripotency and differentiation. In stable cellular conditions, the regulating genes of OCT4, SOX2, and Nanog are inhibited by histone acetylation or deoxyribonucleic acid (DNA) methylation at the promoter regions. Chemicals like 5-azacytidine and valproic acid activate these genes through DNA demethylation or inhibition of histone acetylation.7) Moreover, the transcription factors of OCT4, SOX2, c-Myc, and KLF4 directly generate induced pluripotent stem (iPS) cell formation.3) As an example of the modulation of internal signal transduction molecules, inhibition of bone morphogenetic protein (BMP) induces cardiomyocyte differentiation.8) The known methods to control pluripotency include modulation of extra-cellular growth factors, cytokines, internal signal transduction molecules, and micro ribonucleic acid (microRNA). However, the mechanisms of proliferation and differentiation should be further elucidated. Understanding the biological behavior of stem cells may speed up stem cell therapy.
Embryonic stem (ES) cells have all the requirements of a stem cell: clonality, self-renewal, and multipotentiality. ES cells can completely differentiate into cardiomyocytes or any other type of cell in the human body.9) However, ethical debates have been an obstacle in human application of these cells, because derivation of human ES cells requires destruction of human embryos. Furthermore, ES cells carry the risk of teratoma formation and immune rejection, which disturb transplanted cell function in the recipient body.9) Hence, autologous adult stem cells have attracted attention for cardiovascular therapy. They are free from the risks of teratoma formation and immune rejection, as well as the ethical problems associated with ES cell research.
Stem cells were once believed to differentiate into the cells of the tissue from which they derived. However, transdifferentiation introduced the concept that adult tissue-specific stem cells could differentiate into other lineages. The bone marrow is regarded as a central repository of primitive stem cells that can repopulate somatic tissues. Endothelial progenitor cells (EPCs), bone marrow derived mononuclear cells (BMMNCs), and mesenchymal stem cells (MSCs) are well known the subpopulations of bone marrow derived stem cells that have pooled data associated with human cardiovascular disease.
Orlic et al.10) reported that bone marrow derived stem cells regenerate infarcted myocardium. Human bone marrow derived stem cells have been used in clinical trials during the last decade. Some achievements have been made in human acute myocardial infarction and ischemic cardiomyopathy. The representative human clinical trials are summarized2) in Table 3. All of the studies have shown that human bone marrow derived stem cells are safe for intra-coronary delivery or direct intramyocardial injection.1) Bone marrow derived mononuclear cells, mesenchymal stem cells, and circulating endothelial progenitor cells have been extensively studied.11)
Bone marrow derived stem cells have been shown to promote short-term increases in ejection fraction in the range of 2% to 5% in early non-randomized pilot studies. Post-infarction remodeling was prevented and myocardial perfusion was restored in human trials.11)12) However, larger randomized studies failed to show consistent results, especially in long-term follow-up. In addition, the improvement in ejection fraction was too small to expect that the injected cells were successfully engrafted and contracted synchronously with resident cells. Improvements in post-infarction remodeling and exercise tolerance were superior to those obtained in ejection fraction.13-15)
Murry et al.16) reported that bone marrow derived stem cells do not transdifferentiate into cardiomyocytes in a myocardial infarction animal model. According to the report of Balsam et al.17), the beneficial short-term effects are mostly due to secreted factors (paracrine effect), not to functional engraftment of bone marrow derived stem cells. This paracrine theory is now generally accepted, and new vessel formation and anti-apoptosis are regarded as other mechanisms contributing to functional cardiac improvement.2)
Consensus dictates that functional improvement depends on the salvage of ischemic, jeopardized myocardium. However, fusion of implanted cells with host cardiomyocytes is observed with lower frequency.18) A sub-analysis of the clinical trials indicated that patients with larger or more severe infarctions derive more benefits from stem cell therapy.19) A meta-analysis of pooled data reported that improvements in heart function are significant.20) The long-term follow-up data are expected to provide us more information about therapeutic efficacy.
Endogenous cardiac stem cells (CSCs) do not carry the risk of immune ejection or teratoma formation and are free from the ethical debates associated with the use of ES cells. Cardiac originated progenitor cells are also much more easily accessed for clinical trials and therapeutic applications compared to other types of progenitor cells.
Messina et al.21) identified a clonal cluster called cardiospheres in a suspension culture of a human myocardial tissue biopsy. These cardiospheres consisted of c-Kit+ cells and gave rise to cardiomyocytes, vascular smooth muscles, and endothelial cells when transplanted into an immunodeficient mouse.22) Another cardiac progenitor cell was isolated by the binding affinity to anti-mouse Sca-1 antibody from a human adult heart biopsy. These were called Sca-1+ cells. However, there is debate concerning their cardiac progenicity because these cells require 5-azacytidine demethylation and cardiac fibroblasts to differentiate into cardiomyocytes.23)
Recently, as another progenitor cell source, an endogenous epithelial population has been identified in human epicardium.24) These cells are effective at promoting a short-term increase in ejection fraction in a mouse model. The epicardium may be a novel source of cardiac progenitor cells in human heart. Some reports have shown an association between Isl-1+ cardiac progenitor cells and the epicardium.25) As an animal with epicardial regeneration capacity, the zebrafish is famous for exhibiting spontaneous cardiac regeneration. When the cardiac apex is removed, the pericardial cells are activated, and they replace the lost myocardium by retroprogramming genes to a pluripotent state.26)
To look for optimal endogenous cardiac progenitor cells, researchers are working with human adult heart tissues. However, a definite marker of CSCs has not yet been identified, although c-Kit, Sca-1, and Isl1 are known.27) Furthermore, it remains to be determined if CSCs originate in the heart or are derived from another tissue.
Recently, iPS cells were derived by direct retroprogramming adult endogenous fibroblasts to a pluripotent state.7) In 2006, Takahashi et al.28) reported for the first time that over-expression of four transcription factors (OCT4, SOX2, c-Myc, and KLF4) caused mouse adult fibroblasts to convert into pluripotent stem cells. One year later, Yu29) succeeded in generating human iPS cells from adult skin fibroblasts.
iPS cells show the same characteristics of ES cells in terms of cell surface markers and gene expression. Furthermore, they can differentiate into cells of all three embryonic germ layers.30) Moreover, iPS cells are not associated with an immune rejection or ethical difficulties. Despite survival and safety remain to be addressed, the development of iPS cells presents new and exciting prospects in cell-based therapy for cardiac disease.
Knowledge of stem cell biology will help advance the therapeutic application of stem cells. Such knowledge can be achieved through the coordinated progress of network activities of laboratory stem cell research, technological development, and clinical research. More information will help us determine the most appropriate cell type, cell fate, mechanism of effect, cell modifications, and feasible delivery methods.
The main fields of stem cell research can be divided into cell isolation, manipulation, and survival enhancement, as shown in the schematic flow-chart of stem cell therapy (Fig. 3). Technical development is being pursued to improve delivery systems, tissue engineering, quality assurance, and commercially available cell processing systems. In order for the novel concept of stem cell therapy to be realized, well-regulated, ethically proven, randomized clinical trials are needed to obtain background on the therapeutic mechanisms. The balance between safety and efficacy is the first priority for stem cell therapy to be approved clinically in human disease.
Figures and Tables
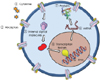 | Fig. 2Schematic diagram of intra-cellular transduction system for pluripotency. The generation of stem cell pluripotency is under genetic regulation. There are several intra-cellular steps occurring between receptor-binding and final protein expression. In laboratory experiments, each step can be modified for a stem cell's pleuripotency. DNA: deoxyribonucleic acid, mRNA: mesenger ribonucleic acid. |
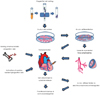 | Fig. 3Schematic flow-diagram of stem cell therapy in cardiac disease. There are three main paths for stem cell application. The process of cell sorting, ex-vivo culture, transplantation, and incorporation represents one path. The process of cell sorting, culture, differentiation, tissue engineering, and transplantation represents another path. The process of activation of resident stem cells in the damaged heart or homing from another organ represents the final path. |
Table 3
Clinical trials of stem cell therapy in human cardiovascular diseases

BMMNC: bone marrow mononuclear cell, DES: drug eluting stent, RCT: randomized clinical trial, c-EPC: circulating endothelial progenitor cell, MSC: mesenchymal stem cell, PBSC: peripheral bone marrow stem cell, EF: ejection fraction, CPC: circulating progenitor cell, CABG: coronary artery bypass surgery, CTO: chronic total occlusion, G-CSF: granulocyte colony-stimulating factor, VT: ventricular tachycardia, VF: ventricular fibrillation
References
1. Passier R, van Laake LW, Mummery CL. Stem-cell-based therapy and lessons from the heart. Nature. 2008. 453:322–329.
2. Jolicoeur EM, Granger CB, Fakunding JL, et al. Bringing cardiovascular cell-based therapy to clinical application: perspectives based on a National Heart, Lung, and Blood Institute Cell Therapy Working Group meeting. Am Heart J. 2007. 153:732–742.
3. Beltrami AP, Urbanek K, Anversa P, et al. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001. 344:1750–1757.
4. Quaini F, Urbanek K, Anversa P, et al. Chimerism of the transplanted heart. N Engl J Med. 2002. 346:5–15.
5. Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci U S A. 1999. 9:10711–10716.
6. Jackson KA, Majka SM, Wang H, et al. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001. 107:1395–1402.
7. Wu SM, Chien KR, Mummery C. Origins and fates of cardiovascular progenitor cells. Cell. 2008. 132:537–543.
8. Yuasa S, Itabashi Y, Koshimizu U, et al. Transient inhibition of BMP signaling by Noggin induces cardiomyocyte differentiation of mouse embryonic stem cells. Nat Biotechnol. 2005. 23:607–611.
9. Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998. 282:1145–1147.
10. Orlic D, Kajstura J, Anversa P, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001. 410:701–705.
11. Wollert KC, Meyer GP, Lotz J, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004. 364:141–148.
12. Schächinger V, Erbs S, Elsässer A, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006. 355:1210–1221.
13. Assmus B, Honold J, Schächinger V, et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006. 355:1222–1232.
14. Adler ED, Maddox TM. Cell therapy for cardiac disease: where do we go from here? Nat Clin Pract Cardiovasc Med. 2007. 4:2–3.
15. Lunde K, Solheim S, Aakhus S, et al. Exercise capacity and quality of life after intracoronary injection of autologous mononuclear bone marrow cells in acute myocardial infarction. Am Heart J. 2007. 154:710.e1–710.e8.
16. Murry CE, Soonpaa MH, Reinecke H, et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004. 428:664–668.
17. Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004. 428:668–673.
18. Nygren JM, Jovinge S, Breitbach M, et al. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med. 2004. 10:494–501.
19. Schächinger V, Tonn T, Dimmeler S, Zeiher AM. Bone-marrow-derived progenitor cell therapy in need of proof of concept: design of the REPAIR-AMI trial. Nat Clin Pract Cardiovasc Med. 2006. 3:Suppl 1. S23–S28.
20. Abdel-Latif A, Bolli R, Tleyjeh IM, et al. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007. 167:989–997.
21. Messina E, De Angelis L, Giacomello A, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004. 95:911–921.
22. Bearzi C, Rota M, Anversa P, et al. Human cardiac stem cells. Proc Natl Acad Sci U S A. 2007. 104:14068–14073.
23. Hudon-David F, Bouzeghrane F, Couture P, Thibault G. Thy-1 expression by cardiac fibroblasts: lack of association with myofibroblast contractile markers. J Mol Cell Cardiol. 2007. 42:991–1000.
24. Smart N, Risebro CA, Melville AA, et al. Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature. 2007. 445:177–182.
25. Lepilina A, Coon AN, Poss KD, et al. A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell. 2006. 127:607–619.
26. Lepilina A, Coon AN, Kikuchi K, et al. A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell. 2006. 127:607–619.
27. Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008. 451:937–942.
28. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006. 126:663–676.
29. Yu J, Vodyanik MA, Thomson JA, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007. 318:1917–1920.
30. Hanna J, Wernig M, Jaenisch R, et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007. 318:1920–1923.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download