Abstract
Eosinophilic endomyocarditis is a manifestation of hypereosinophilic syndrome, characterized by prolonged (>6 months), unexplained peripheral blood eosinophilia (>1,500 cells/mm3) with end-organ damage in unknown causes. We report a case of a 42-year-old patient who developed eosinophilic endomyocarditis following upper respiratory tract symptoms for 2 months. Additionally, endomyocarditis was combined with massive pleural effusion and pericardial effusion, which have not been reported in Korea.
Associations among progressive cardiac failure, eosinophilia, and multi-organ dysfunction were first described by Loeffler in 1936. It was then described as hypereosinophilic syndrome, and diagnostic criteria were proposed as prolonged (>6 months) and unexplained peripheral blood eosinophilia (>1,500 cells/mm3) with end-organ (heart, lung, gastrointestinal tract, brain, skin, bone marrow) damage in unknown causes.1) In such a phenomenon, cardiac manifestation (40-50% of patients) is frequent and damage to the endomyocardium is caused by deposition of toxic granule proteins.2) In Korea, a case of Loeffler's endocarditis with acute obstruction of the common iliac artery was reported in 1999.3)
We report a case of a 42-year-old patient in whom eosinophilic endomyocarditis developed following upper respiratory tract symptoms for 2 months. Additionally, endomyocarditis was combined with massive pleural effusion and pericardial effusion, which have been not reported in Korea.
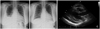
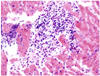
A 42-year-old female patient was admitted to our outpatient clinic with symptoms of pleural chest pain, cough, and dyspnea on exertion. Her medication history included anti-histamine for two months due to upper respiratory symptoms at a primary clinic. Blood pressure was 120/80 mmHg, heart rate was 100 bpm, respiratory rate was 20 per minute, and body temperature was normal. On physical examination, the sound of decreased breathing was observed in the right lower lung. Cardiac auscultation was otherwise normal. Initial laboratory findings revealed hypereosinophilia (660 cells/mm3), with negative parasitological tests and a negative result for Antineutrophil cytoplasmic antibodies (ANCA), with mildly elevated CK-MB (5.23 ng/mL). EKG showed a slightly elevated ST segment in precordial leads (V1, 2, 3, 4). Chest X-ray revealed a right-sided pleural effusion and cardiomegaly (Fig. 1A). Transthoracic echocardiography showed left ventricular hypertrophy and moderate pericardial effusion without thrombus (Fig. 1C). Left ventricular systolic function was in the low to normal range (ejection fraction=51%). Absolute eosinophil count then became elevated to 2,269 cells/mm3; therefore, an endomyocardial biopsy was performed. Biopsy specimens revealed the deposition of degranulated eosinophils in endomyocardium (Fig. 2). A diagnosis of eosinophilic endomyocarditis was thus confirmed, and corticosteroid therapy (prednisolone, 30 mg/day) was planned. One day after the start of medication, absolute eosinophil count was reduced from 2,269 cells/mm3 to 108 cells/mm3, an indication of a good prognosis. Furthermore, follow up transthoracic echocardiography showed that pericardial effusion was reduced, and follow up chest X-ray showed decreased pleural effusion (Fig. 1B). The patient stopped taking prednisolone (2.5 mg/day) after 5 months because absolute eosinophil counts were within normal limits, and follow up echocardiography showed no left ventricular hypertrophy and no residual pericardial effusion. Also the ejection fraction improved from 51% to 77%. At 9-month follow up, the patient was in good condition and no hypereosinophilia was detected.
Eosinophilic endomyocarditis is a subcategory of hypereosinophilic syndrome. Eosinophilic endomyocarditis is caused by direct infiltration of eosinophils into cardiac tissues, so that degranulated eosinophils release cardiotoxic substances, including toxic cationic proteins.4) Acute necrosis, thrombosis, and fibrosis subsequently occurs by these mechanisms.4)
Cardiac evaluation for patients admitted for meeting criteria for eosinophilic endocarditis, should include baseline EKG, echocardiography, and chest X-ray.2) Common findings on the EKG are nonspecific and include ST segment abnormalities.5) Echocardiography often shows ventricular hypertrophy, valvular abnormalities, mural or apical thrombus, and left ventricle (LV) diastolic dysfunction.5) However, despite these noninvasive and newly developed methods, such as cardiac MRI, endomyocardial biopsy is still the gold standard diagnostic method, because other cardiac diseases can be combined with peripheral eosinophilia.2) For example, Churg-strauss syndrome involving the cardiovascular system is almost identical with eosinophilic endomyocarditis in its clinical presentation. Furthermore, once eosinophilic endomyocarditis is confirmed by biopsy, corticosteroids and cytotoxic drugs, such as hydroxyurea could be used effectively. Normalization of absolute eosinophil counts is the first therapeutic goal. Nearly 70% of patients showed a rapid reduction in eosinophils after corticosteroid therapy (which are the drugs of first choice) was administered.1)6)
Typically, endocardium and the underlying myocardium are involved in eosinophilic endomyocarditis.7) However, our patient had concurrent pericardial effusion. Although no pericardial biopsy was performed, pericardial effusion was suggestive of acute pericarditis. In a review of the English literature, there were 55 patients with hypereosinophilic syndrome, with 4% having pericarditis.7) Pericarditis in eosinophilic endomyocarditis is not common, but clinicians should always consider the possibility of pericarditis in eosinophilic endomyocarditis.
Figures and Tables
References
1. Corssmit EP, Trip MD, Durrer JD. Loffler's endomyocarditis in the idiopathic hypereosinophilic syndrome. Cardiology. 1999. 91:272–276.
2. Ogbogu PU, Rosing DR, Horne MK 3rd. Cardiovascular manifestations of hypereosinophilic syndromes. Immunol Allergy Clin North Am. 2007. 27:457–475.
3. Shin SK, Lee DH, Hur SH, et al. A case of Loeffler's endocarditis with acute obstruction of common iliac artery. Korean Circ J. 1999. 29:1264–1270.
4. Tai PC, Ackerman SJ, Spry CJ, Dunnette S, Olsen EG, Gleich GJ. Deposits of eosinophil granule proteins in cardiac tissues of patients with eosinophilic endomyocardial disease. Lancet. 1987. 1:643–647.
5. Braunwald E, Zipes DP, Libby P. Heart Disease: A Textbook of Cardiovascular Medicine. 2001. Philadelphia: WB Saunders.
6. Parrillo JE, Fauci AS, Wolff SM. Therapy of the hypereosinophilic syndrome. Ann Intern Med. 1978. 89:167–172.
7. Parrillo JE, Borer JS, Henry WL, Wolff SM, Fauci AS. The cardiovascular manifestations of the hypereosinophilic syndrome: prospective study of 26 patients, with review of the literature. Am J Med. 1979. 67:572–582.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download