Abstract
Background and Objectives
Clopidogrel resistance or low-responsiveness may be associated with recurrent atherothrombotic events after drug-eluting stent (DES) implantation. We prospectively evaluated the association between clopidogrel resistance assessed by the Verifynow™ P2Y12 assay (Accumetrics, San Diego, CA, USA) and stent thrombosis (ST) or cardiac death (CD) in patients with acute coronary syndrome (ACS) within 6 months after DES implantation.
Subjects and Methods
We enrolled 237 consecutive patients (160 males, 65.2±10.3 years) with ACS who received a DES implantation. The composite endpoint was defined to CD or ST by Academic Research Consortium definitions within 6 months post-implantation. Clopidogrel resistance was defined as <20% inhibition of the P2Y12 receptor.
Results
Baseline demographic characteristics were similar between 142 normal individuals and 95 clopidogrel resistant patients. CD occurred in one case (0.7%) in the normal group and two cases (2.13%) in the resistant group (p=0.344). There was no episode of ST in the normal group and four episodes in the resistant group (4.2%, four definite ST) (p=0.035). Univariate logistic regression revealed an adjusted odds ratio (OR) for composite end point of CD or ST of 9.646 {95% confidence interval (CI) 1.139-81.679}, and multivariate logistic regression for composite end point revealed an OR of 12.074 (95% CI 1.205-120.992).
Six months following percutaneous coronary artery intervention (PCI), coronary angiography has revealed a re-stenosis rate of 30-40% in patients treated using balloon dilatation and 20-30% in those treated using a bare metal stent.1)2) In recent years, use of drug-eluting stents (DESs) has lowered the re-stenosis rate by <10%, and the need for repeated PCI due to re-stenosis has also declined, which have prompted the marked increase in the use of DESs.3) PCI is also performed in cases in which there are small-sized, long lesions, as well as for treatment of multivessel diseases.4-6) In recent years, however, the occurrence of stent thrombosis (ST) associated with DESs has been increasingly reported. ST can prelude a poor outcome such a acute myocardial infarction (MI) or sudden cardiac death (CD).7) In an actual clinical setting, the frequency has been reported to be higher than that reported on well-designed largescale clinical trials. Furthermore, an association of ST and early discontinuation of antiplatelet agents has been reported. The United States Food and Drug Administration has recommended that antiplatelet agents such as aspirin or clopidogrel be used for at least one year following the use of a DES.8-10)
Recent studies have shown that anti-platelet responses to aspirin and clopidogrel vary depending on patients. In the studies, appropriate anti-platelet responses were not achieved in 5-45% of patients taking aspirin or 4-30% of those taking clopidogrel.11-14) In the study, approximately 25% of patients with ST-segment elevation MI had a resistance to clopidogrel, in whom the incidence of cardiovascular complications has been reported to increase after a 6-month period.15-17)
Methods for measuring clopidogrel resistance include light transmittance aggregometry (LTA), vasodilator-stimulated phosphoprotein (VASP) phosphorylation assay, use of the Platelet Function Analyzer-100™ and the Verify-Now™ P2Y12 assay (Accumetrics, San Diego, CA, USA). LTA is a standardized measurement method, but it cannot be generally used because special training is needed and its manipulation is complicated. By contrast, the point-of-care VerifyNow™ P2Y12 assay is a simpler means of measurement that produces results nearly identical to those of LTA, prompting its widespread use.18-20) Many studies have shown that patients with LTA-determined clopidogrel resistance are at greater risk for cardiovascular death (CVD) following PCI using a DES.17)21-24) However, the relationship between CVD and clopidogrel resistance measured using the VerifyNow™ P2Y12 assay is unclear, and is completely unknown in patients with acute coronary syndrome (ACS).
Presently, we attempted to examine the effect of clopidogrel resistance measured using the VerifyNow™ P2Y12 assay on ST or CD within 6 months following the procedure in patients with ACS who underwent PCI using a DES.
The current study was a single-center, prospective, cohort study conducted in 237 patients (age 65.2±10.3 years, of whom 160 were male), in whom the anti-platelet effects of clopidogrel were confirmed by the VerifyNow™ P2Y12 assay. The patients had been diagnosed with ACS (unstable angina or non-ST-segment elevated MI), and had undergone coronary angiography involving the insertion of a DES due to >50% coronary artery stenosis. All procedures were conducted at the Department of Cardiology, Wonju Christian Hospital, from January 2006 to December 2007. Exclusion criteria were renal failure, presence of an infectious disease, left ventricular ejection fraction <30%, malignant neoplasm, hepatic failure, previous long-term use of anticoagulation drugs, history of hemorrhagic disease and platelet counts <150,000 per mL.
For the assessment of clopidogrel responsiveness, samples were collected from the vein following PCI and 5 days after initiating regular administration of clopidogrel (75 mg). Each sample was placed in a tube containing 3.2% citrate and clopidogrel resistance was assessed within 8 hours. Sampling was done in the hospital for patients who were continually hospitalized during this period or during the first follow-up for patients who had been discharged. Clopidogrel resistance was determined as detailed below.
Clopidogrel non-response (resistance) is considered to be a degree of responsiveness ≤10%, while semires-ponse ranges from 11% to <30%.26)27) Resistance can also be determined with the value corresponding to the 4th quartile based on four quartiles.17)
In a preliminary study conducted at Wonju Christian Hospital, 500 patients were tested. The inhibition rate (% inhibition) for P2Y12 receptor was 20%, which corresponded to the value of the 4th quartile. Accordingly, in the current study, patients whose inhibition rate for P2Y12 receptor was <20% were determined to have a clopidogrel resistance. For cases in which the responses to clopidogrel were assessed using the VerifyNow™ system, an inhibition rate for the P2Y12 receptor ≥20% was defined as the normal control group (normal response) and otherwise cases were defined as the resistance group (low response). Currently, there are no established guidelines for the treatment of resistance group. Therefore, clopidogrel was continually used and was not replaced by other drugs.
The primary endpoint was ST or CD that occurred 6 months following the insertion of a DES. The composite end point was cases in which ST or CD occurred.
ST was classified as 'definite', 'probable' and 'possible' according to the definition of Academic Research Consortium (ARC). Based on the phase, it was also classified as acute, subacute or late. Definite ST was defined in patients who displayed ischemic chest pain and its concurrent presence with acute ischemic change in an electrocardiogram, and patients who showed an elevated level of the cardiac enzymes in cases in which an incomplete or complete obstruction of the blood vessels were present due to the presence of in-stent thrombosis on coronary angiography. Probable ST was defined as a diagnosis of MI confirmed around the area of stent insertion or in cases in which death due to an unknown cause occurred within 30 days post-PCI. Possible ST was defined as cases in which idiopathic sudden death occurred within 30 days post-PCI. Acute, subacute and late ST were defined as cases in which ST occurred within 24 hours, 1 day-1 month and more than one month following PCI, respectively.
For a stent insertion, after the radial or femoral artery was punctured, a 6-7 Fr sheath was inserted. Through an arterial sheath, a catheter was inserted. All the procedures were performed using a catheter and a guide wire. In most cases, a stent insertion was performed for cases in which the remaining stenosis was present following a balloon dilatation of the coronary artery. A determination on stent insertion was wholly dependent on the subjective judgment of clinicians. Successful stent insertion was defined as cases in which residual stenosis was <30% following stent insertion. In cases in which a residual stenosis remained following a stent insertion, with the additional use of a balloon dilatation, the procedures were performed in such a manner as to minimize the residual stenosis. Prior to stent insertion, all the patients received a pre-treatment using 600 mg clopidogrel and 300 mg aspirin. In patients who received a DES, 100 mg aspirin was administered for life and 75 mg clopidogrel was administered for at least 6 months in principle.
Data is expressed as mean±standard deviation. Statistical analysis was performed using Statistical Package for Social Science (SPSS) software for Windows, version 12.0 (SPSS Inc., Chicago, IL, USA). The current study examined the frequency of ST. The Chi-square test and Fisher's exact test assessed whether the frequency of ST would vary depending on the responsiveness of receptor to clopidogrel. To analyze the odds ratio (OR) of developing ST and CD, binary logistric regression and a multivariable logistic regression were used. A p of <0.05 was considered statistically significant.
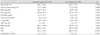
Of patients with ACS who received a DES, the antiplatelet effects of clopidogrel were measured using the VerifyNow™ P2Y12 assay in 237 patients (65.2±10.3 years, including 160 male patients). Of the 142 patients of the normal control group (63.9±10.9 years, including 94 male patients), 43 (30.3%) had a non-ST-segment elevation MI and the remaining patients had an unstable angina. Of the 95 patients defined with clopidogrel resistance (64.8±11.1 years, including 66 male patients), 32 (33.7%) had a non-ST-segment elevation MI and the remaining patients had an unstable angina. There was no significant difference between the two groups (p=0.581). In addition, there were no significant differences between the groups in the number of patients who had MI, diabetes mellitus, hypertension, smoking or aspirin resistance (Table 1). There were no significant differences in hematologic findings between the two groups. But there was a significant difference in the inhibition rate for P2Y12 receptor and P2Y12 reaction unit (PRU) between the two groups (Table 2).
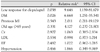
ST never developed in the normal control group and occurred in four patients of the resistance group. The difference reached statistical significance (p=0.014) (Table 3). ST in the resistance group comprised one acute case, one subacute case and two late cases; all were definite ST. CD occurred in one patient of the normal control group and two patients of the resistance group, and was not significant (p=0.344). When the incidences of ST or CD were summed, they were revealed in one patient of the normal control group and six patients of the resistance group. This difference reached statistical significance (p=0.012) (Table 3). In the resistance group, there were four cases of ST and two cases of CD (Fig. 1).
Univariate analysis based on a binary logistic regression analysis showed that the OR of clopidogrel resistance in the development of ST or CD was 9.646 {95% confidence interval (CI); 1.139-81.679}. A multivariate analysis performed based on a multivariate logistic regression analysis showed that the OR of clopidogrel resistance in developing ST or CD was 12.074 (95% CI; 1.205-120.992) (Table 4 and 5).
The incidence of ST, which has previously been reported in well-designed large-scale clinical trials was not relatively higher presently. ST has been reported to be similar to that seen following the use of a bare-metal stent. However, application of the results of well-designed large-scale clinical trials to actual clinical settings remains problematic. Clinical studies are mostly conducted in patients who are eligible for specific selection criteria and tend to exclude unstable status of patients with cardiac necrosis due to thrombotic occlusion events such as MI, PCI performed in an emergency setting and presence of a complex lesion such as bifurcation lesion or chronic total occlusion. It can, therefore, be inferred that the frequency of ST will likely not be relatively higher following the use of DESs in a real world setting. In an actually clinical setting, it would be markedly higher than the incidence of ST, which has been reported on well-designed large-scale clinical trials.28)
Recent studies have shown that the incidence of STs is increased in the clinical setting.7,29-31) Indeed, in a realworld setting, prognostic indicators for predicting ST include the early discontinuation of use of antiplatelet agents, renal failure, bifurcation lesion, diabetes mellitus and a lower level of left ventricular ejection fraction.29) A recent study using intravascular ultrasound has shown that optimal inflation of a stent is not associated with prognosis.32) It has also been reported, however, that incomplete inflation of a stent and a significant residual stenosis rate may be associated with the occurrence of ST.33) Of these prognostic indicators, early discontinuation of use of antiplatelet agents has been reported to play a major role. Clinically, a low clopidogrel responsiveness has a similar risk profile as compared with the early discontinuation of use of antiplatelet agents. Clopidogrel resistance measured using LTA following stent insertion showed a relative risk of 3.71 (95% CI; 1.08-12.69) for CVD.24) Clopidogrel resistance measured using Platelet Function Analyzer-100 showed a relative risk of 2.5 (95% CI; 1.6-3.8) for hospitalization due to CD and MI. In addition, clopidogrel resistance has been reported to play a role in achieving a longterm prognosis as one of the independent prognostic indicators.34) In a previous study that measured the relationship of clopidogrel resistance determined using the VerifyNow™ system, with the occurrence of CVD and ST in 380 patients (93.7%) with stable angina, event-free survival was significantly lower in the resistance group (91.5% vs. 99.0%, p=0.004).35)
The current study also measured the responsiveness to clopidogrel using the VerifyNow™ system, with the aim of clarifying the relationship between CD and ST. In particular, the current study enrolled patients with ACS, a subject patient group for which only a small number of studies have been conducted, and then performed a follow-up study in an outpatient setting concomitantly with a follow-up telephone survey. The current study examined all the patients who were suspected to have a ST. Our univariate and multivariate analyses strongly implicate clopidogrel resistance as an independent prognostic indicator for predicting the occurrence of ST or CD. These results agree with those obtained by LTA and the Platelet Function Analyzer-100. As shown in patients with stable angina, the present data indicates a key role for clopidogrel resistance in those with ACS.
A noteworthy clinical point in the current study was the finding that all the cases of ST occurred within 6 months following the procedure, during which patients were taking both aspirin and clopidogrel. This may indicate that ST cannot be prevented using a maintenance therapy with aspirin and clopidogrel. For the prevention of ST, the United States Food and Drug Administration recommends that a maintenance therapy with aspirin and clopidogrel be performed for at least 1 year in patients who receive a DES.8-10) But, maintenance therapy might not be effective in completely preventing the occurrence of ST, with more active therapy and management being necessary.
The present study has two limitations. Firstly, we did not completely evaluate patient-related risk factors of developing ST (e.g., local activity of platelet aggregation), lesion-related risk factors (e.g., length of lesions and characteristics of artherosclerotic plaques) and procedural risk factors (e.g., incomplete inflation of a stent). It could be inferred, however, that relatedness diminished lesion-related and procedure-related risk factors due to the common use of intravascular ultrasound and high pressure balloon. By contrast, it could also be inferred that patient-related risk factors such as clopidogrel resistance would assume greater importance. The second limitation concerns the study size; although the total number of enrolled patients was 273, only seven patients presented with ST or CD. Despite the presence of statistical significance, the possibility for a bias due to a smaller sample size cannot be excluded.
Despite these limitations, the current study is of clinical significance in that it provides a basis by which drug augmentation or new drugs could be used in the event of PCI with a DES, since risk factors of developing ST or CD using point-of-care system were stratified. As with other medical advances, further large-scale prospective studies are necessary.
Figures and Tables
Table 1
Baseline characteristics of study patients

Aspirin resistance: ARU ≥550 (aspirin reaction unit). NSTEMI: non-ST elevation myocardial infarction, CVA: cerebrovascular accident, MI: myocardial infarction, DM: diabetes mellitus, PCI: percutaneous coronary intervention, LAD: left anterior descending artery, LCX: left circumflex artery, RCA: right coronary artery, LM: left ma in
Table 3
Difference between normal and low response of clopidogrel for stent thrombosis (ST) and cardiac death

References
1. Serruys PW, de Jaegere P, Kiemeneij F, et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent Study Group. N Engl J Med. 1994. 331:489–495.
2. Fischman DL, Leon MB, Baim DS, et al. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study Investigators. N Engl J Med. 1994. 331:496–501.
3. Babapulle MN, Joseph L, Belisle P, Brophy JM, Eisenberg MJ. A hierarchical Bayesian meta-analysis of randomised clinical trials of drug-eluting stents. Lancet. 2004. 364:583–591.
4. Schampaert E, Cohen EA, Schluter M, et al. The Canadian study of the sirolimus-eluting stent in the treatment of patients with long de novo lesions in small native coronary arteries (C-SIRIUS). J Am Coll Cardiol. 2004. 43:1110–1115.
5. Schofer J, Schluter M, Gershlick AH, et al. Sirolimus-eluting stents for treatment of patients with long atherosclerotic lesions in small coronary arteries: double-blind, randomised controlled trial (E-SIRIUS). Lancet. 2003. 362:1093–1099.
6. Park SJ, Kim YH, Lee BK, et al. Sirolimus-eluting stent implantation for unprotected left main coronary artery stenosis: comparison with bare metal stent implantation. J Am Coll Cardiol. 2005. 45:351–356.
7. Jeremias A, Sylvia B, Bridges J, et al. Stent thrombosis after successful sirolimus-eluting stent implantation. Circulation. 2004. 109:1930–1932.
8. De Luca G, Carbone G, Maione A, Gregorio G. In-stent thrombosis after discontinuation of antiplatelet therapy 2 years after DES implantation: a case report. Int J Cardiol. 2007. 116:399–400.
9. Artang R, Dieter RS. Analysis of 36 reported cases of late thrombosis in drug-eluting stents placed in coronary arteries. Am J Cardiol. 2007. 99:1039–1043.
10. Grines CL, Bonow RO, Casey DE Jr, et al. Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians. Catheter Cardiovasc Interv. 2007. 69:334–340.
11. Eikelboom JW, Hirsh J, Weith JI, et al. Aspirin-resistant thromboxane biosynthesis and the risk of myocardiac infarction, stroke or cordiovascular death in patients at high risk for cardiovascular events. Circulation. 2002. 105:1650–1655.
12. Gum PA, Kottke-Morchant K, Poggio ED, et al. Profile and prevalence of aspirin resistance in patients with cardiovascular disease. Am J Cardiol. 2001. 88:230–235.
13. Gusbel PA, Bilden KP, Hiatt BL, et al. Clopidogrel for coronary stenting: response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation. 2003. 107:2908–2913.
14. Serebruary VL, Steingubl SR, Berger PB, et al. Variability in platelet responsivent to clopidogrel among 544 individuals. J Am Coll Cordiol. 2005. 45:246–251.
15. Cutlip DE, Baim DS, Ho KK, et al. Stent thrombosis in the modern era: a pooled analysis of multicenter coronary stent clinical trials. Circulation. 2001. 103:1967–1971.
16. Wiviott SD, Antman EM. Clopidogrel resistance: a new chapter in a fast-moving story. Circulation. 2004. 109:3064–3067.
17. Matetzky S, Shenkman B, Guetta V, et al. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation. 2004. 109:3171–3175.
18. van Werkum JW, van der Stelt CA, Seesing TH, Hackeng CM, ten Berg JM. A head-to-head comparison between the VerifyNow P2Y12 assay and light transmittance aggregometry for monitoring the individual platelet response to clopidogrel in patients undergoing elective percutaneous coronary intervention. J Thromb Haemost. 2006. 4:2516–2518.
19. Malinin A, Pokov A, Swaim L, Kotob M, Serebruany V. Validation of a VerifyNow-P2Y12 cartridge for monitoring platelet inhibition with clopidogrel. Methods Find Exp Clin Pharmacol. 2006. 28:315–322.
20. Paniccia R, Antonucci E, Gori AM, et al. Different methodologies for evaluating the effect of clopidogrel on platelet function in high-risk coronary artery disease patients. J Thromb Haemost. 2007. 5:1839–1847.
21. Lev EI, Patel RT, Maresh KJ, et al. Aspirin and clopidogrel drug response in patients undergoing percutaneous coronary intervention: the role of dual drug resistance. J Am Coll Cardiol. 2006. 47:27–33.
22. Gurbel PA, Bliden KP, Zaman KA, Yoho JA, Hayes KM, Tantry US. Clopidogrel loading with eptifibatide to arrest the reactivity of platelets: results of the Clopidogrel Loading With Eptifibatide to Arrest the Reactivity of Platelets (CLEAR PLATELETS) study. Circulation. 2005. 111:1153–1159.
23. Cuisset T, Frere C, Quilici J, et al. High post-treatment platelet reactivity identified low-responders to dual antiplatelet therapy at increased risk of recurrent cardiovascular events after stenting for acute coronary syndrome. J Thromb Haemost. 2006. 4:542–549.
24. Geisler T, Langer H, Wydymus M, et al. Low response to clopidogrel is associated with cardiovascular outcome after coronary stent implantation. Eur Heart J. 2006. 27:2420–2425.
25. Gurbel PA, Bliden KP, Guyer K, et al. Platelet reactivity in patients and recurrent events post-stenting: results of the PREPARE POST-STENTING Study. J Am Coll Cardiol. 2005. 46:1820–1826.
26. Samara WM, Bliden KP, Tantry US, Gurbel PA. The difference between clopidogrel responsiveness and posttreatment platelet reactivity. Thromb Res. 2005. 115:89–94.
27. Buonamici P, Marcucci R, Migliorini A, et al. Impact of platelet reactivity after clopidogrel administration on drug-eluting stent thrombosis. J Am Coll Cardiol. 2007. 49:2312–2317.
28. Farb A, Boam AB. Stent thrombosis redux: the FDA perspective. N Engl J Med. 2007. 356:984–987.
29. Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drugeluting stents. JAMA. 2005. 293:2126–2130.
30. Park DW, Park SW. Stent thrombosis in the era of the drug-eluting stent. Korean Circ J. 2005. 35:791–794.
31. Park S, Hong GR, Seo HS, Tahk SJ. Stent thrombosis after successful drug-eluting stent implantation. Korean Circ J. 2005. 35:163–171.
32. Nam CW, Kim KB, Hur SH, et al. Impact of optimal stent expansion on late outcomes after sirolimus-eluting stent implantation: an intravascular ultrasound study. Korean Circ J. 2007. 37:244–250.
33. Fujii K, Carlier SG, Mintz GS, et al. Stent underexpansion and residual reference segment stenosis are related to stent thrombosis after sirolimus-eluting stent implantation: an intravascular ultrasound study. J Am Coll Cardiol. 2005. 45:995–998.
34. Foussas SG, Zairis MN, Patsourakos NG, et al. The impact of oral antiplatelet responsiveness on the long-term prognosis after coronary stenting. Am Heart J. 2007. 154:676–681.
35. Price MJ, Endemann S, Gollapudi RR, et al. Prognostic significance of post-clopidogrel platelet reactivity assessed by a pointof-care assay on thrombotic events after drug-eluting stent implantation. Eur Heart J. 2008. 29:992–1000.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download