Abstract
Isolated left ventricular noncompaction cardiomyopathy (IVNC) is a cardiomyopathy thought to be caused by arrest of normal embryogenesis of the endocardium and myocardium. This abnormality is often associated with other congenital cardiac defects. A 21-year-old man presented to the emergency department with worsening exertional dyspnea during the previous 2 months. Two-dimensional and Doppler echocardiography revealed an enlarged left atrium (LA) and a markedly dilated left ventricle (LV) with preserved LV systolic function, severe mitral valve regurgitation, and prolapse due to chordae rupture. The myocardium of the LV and right ventricle (RV) had excessively prominent trabeculations and deep intertrabecular recesses. He is the first patient in Korea who has undergone mitral valve replacement surgery because of severe mitral valve regurgitation and prolapse due to chordae rupture accompanied by IVNC.
Isolated left ventricular noncompaction cardiomyopathy (IVNC) is a congenital cardiomyopathy caused by a defect in endomyocardial morphogenesis.1) Although IVNC has been a known clinical entity for more than a decade, IVNC is still an unclassified cardiomyopathy according to the World Health Organization classification of cardiomyopathies.2) The major clinical manifestations of IVNC are depressed left ventricular systolic and diastolic function, systemic embolism, ventricular tachyarrhythmias, conduction disorders, and neurologic abnormalities.3)4) Medical management of heart failure and heart transplantation, when clinically indicated, management of arrhythmias, prevention of systemic embolism, and echocardiographic screening of first-degree relatives are accepted treatment strategies.3)5-7) We report patient with IVNC accompanied by severe mitral valve regurgitation and prolapse due to chordae rupture who was treated successfully with mitral valve replacement surgery and tricuspid annuloplasty.
A 21-year-old man was admitted to our emergency room because of worsening exertional dyspnea during the previous 2 months. He had experienced shortness of breath on climbing stairs or during military drills. The frequency of dyspnea had recently increased. The patient did not smoke cigarettes or drink alcohol and had none of the classic cardiovascular risk factors. A physical examination revealed jugular venous distension and the carotid pulses were rapid without bruits. The patient's arterial pressure was 110/60 mmHg, the body temperature was 37.8℃, the respiratory rate was 22/min, and the heart rate was 110 bpm. Cardiac auscultation revealed a regular and rapid rhythm, and a grade 4/6 pansystolic murmur at the cardiac apex with a palpable thrill. The lungs were clear to auscultation. There was no hepatomegaly, splenomegaly, or lower extremity pitting edema. The electrocardiography (ECG) in the emergency room showed sinus tachycardia without ST changes. The chest radiography demonstrated marked cardiomegaly with left atrial cardiac border straightening and clear lung fields. The results of routine hematologic and biochemical parameters, including cardiac enzymes, were within the normal range. Two-dimensional and Doppler echocardiography (Vivid 7 ultrasound scanner; General Electric, Milwaukee, WI, USA) revealed an enlarged left atrium (LA; 50 mm) and a markedly dilated left ventricle (LV; 68 mm) with preserved LV systolic function {ejection fraction (EF)=50%}, severe mitral valve regurgitation, and prolapse due to chordae rupture (Fig. 1). There was also mild tricuspid valve regurgitation and severe pulmonary hypertension {tricuspid valve regurgitant maximal velocity (TR Vmax)=3.72 m/s; right ventricular systolic pressure (RVSP)=75.31 mmHg} with a dilated and plethoric inferior vena cava (21.6 mm). The right atrial (RA) and ventricular size and morphology, as well as the function of the aortic and pulmonary valves, were normal. There was no pericardial effusion. On the apical 4-chamber view, the myocardium of the LV and right ventricle (RV) had excessively prominent trabeculations and deep intertrabecular recesses at the LV apex and mid-ventricular segment of the inferior and lateral wall. The affected segments had a 2-layer structure (a compact epicardial layer and an endocardial layer consisting of a prominent trabecular meshwork and deep intertrabecular spaces) (Fig. 2). The ratio of the noncompacted-to-compacted myocardial layers at the site of the maximal wall thickness was 3.84. On color Doppler analysis, intertrabecular spaces were filled by direct blood flow from the ventricular cavity throughout the cardiac cycle. On contrast echocardiogram (DEFINITY®; Lantheus Medical Imaging, Inc., North Billerrca, MA, USA), the apical long-axis view showed prominent ventricular trabeculations and deep intertrabecular recesses (Fig. 3). The mitral inflow pattern was consistent with restrictive physiology (mitral E velocity, 178.28 cm/s; mitral A velocity, 77.45 cm/s; and deceleration time, 85.68 ms).
We performed coronary angiography, cardiac catheterization, and left ventriculography to exclude other coexisting cardiac abnormalities. The coronary vessels were normal on coronary angiography and heart catheterization revealed moderately elevated chamber pressures (pulmonary capillary wedge pressure=40 mmHg; pulmonary artery pressure=65 mmHg; RV pressure=50 mmHg; and RA pressure=10 mmHg). The left ventriculogram was consistent with isolated noncompaction of the ventricular myocardium and severe mitral regurgitation by angiography (Fig. 4). The T1-weighted black blood axial and cine two-chamber magnetic resonance images (MRIs) showed a trabecular meshwork and deep intertrabecular recesses in the LV and wall (Fig. 5).
Echocardiographic screening of the first-degree relatives was also performed. The patient's mother was shown to have a LV with muscular trabeculations and deep invaginations. Surgery was performed through a median sternotomy and the patient was placed on cardiopulmonary bypass. On gross findings of the LV cavity, the anterior mitral leaflet chordae were ruptured and severe noncompacted endomyocardium was observed. Owing to a friable posterior lateral papillary muscle, a mitral valve repair could not be performed. Thus, the anterior mitral leaflet was resected (Fig. 6) and replaced with a St. Jude mechanical 29 mm valve. The posterior mitral leaflet and the chordae were preserved. The tricuspid annuloplasty with a flexible ring was completed. He was discharged in very good condition without exertional dyspnea.
Noncompaction of the ventricular myocardium, which was first described in 1984 by Engberding and Bender,8) is a rare congenital cardiomyopathy characterized by multiple prominent trabeculations with deep intertrabecular recesses resulting from an arrest in normal embryogenesis of the endocardium and myocardium. This abnormality can either be isolated, as first described by Chin et al.1) in 1990, or associated with complex congenital heart disease. IVNC is the result of an arrest in the normal process of myocardial compaction, and is characterized by persistence of multiple, excessively prominent ventricular trabeculations and deep intertrabecular recesses.9) IVNC is a genetically heterogeneous disorder. A proper diagnosis of IVNC is crucial not only because of its high mortality in symptomatic patients, but also for screening relatives, as familial occurrence is known. Both familial and sporadic forms have been described.10) Adult forms of IVNC are genetically distinct from X-linked infantile cases in that mutations in the G 4.5 gene have not been shown to be the underlying mechanism. Adult forms of IVNC have been suggested to be transmitted by an autosomal dominant trait.11) This is based on the observations that about one-half of descendants of patients with IVNC inherit the condition. Furthermore, there are cases of male-to-male transmission and the disorder may occur in females also. IVNC in adults is a distinct entity without the extracardiac manifestations that occur in children. Interestingly, familial recurrence seems to be more common in adult patients with IVNC11) than in pediatric populations.1) Echocardiographic examinations of the first-degree relatives of our case revealed that the patient's mother was also affected, but she had no clinical symptoms. There are some case reports involving the adult form of IVNC in Korea;12-14) however, there have been no case reports of IVNC accompanied by severe mitral regurgitation and prolapse due to chordae rupture. The triad of heart failure symptoms, arrhythmias, and embolic events is the major clinical manifestation in patients with reduced systolic left ventricular function15) and is comparable in adult and pediatric populations.1) Various patterns of arrhythmias, ranging from atrial fibrillation to sustained ventricular tachycardia, can be observed. Our case's patient was also admitted with symptoms of severe left ventricular systolic and diastolic dysfunction, no history of systemic embolization was present, and we were able to document a non-sustained ventricular tachycardia with 24-hour Holter monitoring during the hospitalization period. Echocardiography is considered the reference standard for the diagnosis of IVNC. To standardize and facilitate the diagnosis, Jenni et al.9) established four clear-cut echocardiographic criteria. A potential advantage of MRI is the possibility of identifying subendocardial perfusion deficits.16) Although echocardiography is the reference standard for the diagnosis of IVNC, MRI clearly has potential, especially in patients in whom good echocardiographic quality cannot be obtained.
In a larger series of patients,17) long-term follow-up (39 patients over 44 months) showed that 35% died, one-half of them because of sudden cardiac death, and 12% (four patients) had undergone heart transplantation. The high incidence of both thromboembolic events (24%) and ventricular tachycardia (41%) in that series underscores the poor clinical prognosis for patients with impaired left ventricular function. Certain clinical characteristics have been found to be significantly more common in non-survivors than in long-term survivors of IVNC17) (higher left ventricular end diastolic diameter at the time of initial presentation, New York Heart Association class III/IV, chronic atrial fibrillation, and bundle branch block). It has not been reported that a relationship between myxomatous mitral valve regurgitation and IVNC exists. Management of patients with IVNC is similar to that of patients with other cardiomyopathies and should therefore include appropriate treatment for heart failure, management of arrhythmias, and oral anticoagulation to prevent systemic emboli in patients with impaired left ventricular function.10) Implantation of an internal cardioverter defibrillator system and early listing of symptomatic patients for heart transplantation must be seriously considered.10) Thus, we performed a mitral valve replacement and tricuspid annuloplasty. Fortunately, he has improved significantly post-operatively. We presumed that the cause of his dyspnea was progressive mitral valve regurgitation by chordae rupture, rather than LV systolic dysfunction by IVNC. Because of the high mortality rate, patients with IVNC should be followed-up closely with appropriate medical management and considered for heart transplantation with a high priority. With all these data, he is presented as a case report because he is the first IVNC patient in Korea who underwent mitral valve replacement surgery because of severe mitral valve regurgitation and prolapse due to chordae rupture.
Figures and Tables
 | Fig. 1A: on apical 4-chamber view, color flow imaging shows a large proximal isovelocity surface area and mitral regurgitant flow. B: two-dimensional echocardiogram demonstrates chordae rupture of the anterior mitral leaflet (white arrow). LA: left atrium, LV: left ventricle. |
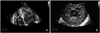 | Fig. 2A: on apical 4-chamber view, the LV and RV myocardium have excessively prominent trabeculations and deep intertrabecular recesses. B: parasternal short axis view at the mid-ventricular level shows a loosened spongy myocardium. LV: left ventricle, RV: right ventricle. |
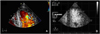 | Fig. 3A: on color flow imaging, intertrabecular spaces are filled by direct blood flow from the ventricular cavity. B: contrast echocardiogram shows prominent ventricular trabeculations and deep intertrabecular recesses at the apicolateral wall. LV: left ventricle. |
 | Fig. 4Left ventriculogram of a patient with isolated left ventricular noncompaction. The spongy-like appearance of the noncompacted ventricular wall during the diastolic phase is seen. Angiographic severe mitral regurgitation and hypokinesis of the noncompacted ventricular wall are also seen. |
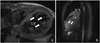 | Fig. 5T1-weighted black blood axial (A) and cine two-chamber (B) magnetic resonance images of a patient with isolated left ventricular noncompaction cardiomyopathy. There are numerous, excessively prominent trabecular meshwork and deep intertrabecular recesses that penetrated deeply into the left ventricular wall (white arrow). LV: left ventricle. |
References
1. Chin TK, Perloff JK, Williams RG, Jue K, Mohrmann R. Isolated non-compaction of left ventricular myocardium: a study of eight cases. Circulation. 1990. 82:507–513.
2. Richardson P, McKenna W, Bristow M, et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the definition and classification of cardiomyopathies. Circulation. 1996. 93:841–842.
3. Agmon Y, Connolly HM, Olson LJ, Khadheria BK, Seward JB. Noncompaction of the ventricular myocardium. J Am Soc Echocardiogr. 1999. 12:859–863.
4. Pignatelli R, McMahon JC, Dreyer WJ, et al. Clinical characterization of left ventricular noncompaction in children: a relatively common form of cardiomyopathy. Circulation. 2003. 108:2672–2678.
5. Weiford BC, Subbarao VD, Mulhern KM. Noncompaction of the ventricular myocardium. Circulation. 2004. 109:2965–2971.
6. Stamou SC, Lefrak EA, Athari FC, Burton NA, Massimiano PS. Heart transplantation in a patient with isolated noncompaction of the left ventricular myocardium. Ann Thorac Surg. 2004. 77:1806–1808.
7. Conraads V, Paelinck B, Vorlat A, Goethals M, Jacobs W, Vrints C. Isolated non-compaction of the left ventricle: a rare indication for transplantation. J Heart Lung Transplant. 2001. 20:904–907.
8. Engberding R, Bender F. Identification of a rare congenital anomaly of the myocardium by two-dimensional echocardiography: persistence of isolated myocardial sinusoids. Am J Cardiol. 1984. 53:1733–1734.
9. Jenni R, Goebel N, Tartini R, Schneider J, Arbenz U, Oelz O. Persisting myocardial sinusoids of both ventricles as an isolated anomaly: echocardiographic, angiographic, and pathologic anatomical findings. Cardiovasc Intervent Radiol. 1986. 9:127–131.
10. Jenni R, Oechslin EN, van der Loo B. Isolated ventricular noncompaction of the myocardium in adults. Heart. 2007. 93:11–15.
11. Sasse-Klaassen S, Gerull B, Oechslin E, Jenni R, Thierfelder L. Isolated noncompaction of the left ventricular myocardium in the adult is an autosomal dominant disorder in the majority of patients. Am J Med Genet A. 2003. 119A:162–167.
12. Lee SW, Koh MB, Hur WH, et al. A case of spongy myocardium initially manifested by ventricular tachycardia in adult. Korean J Med. 2003. 65:Suppl 3. S733–S737.
13. Kim WS, Mang JH, Park SJ, et al. Two case of spongy myocardium detected in adult. J Korean Soc Echocardiogr. 2003. 11:108–113.
14. Lim YJ, Kim JS, Kim IH, et al. A case of isolated noncompaction of the ventricular myocardium in an elderly patient. Korean J Med. 2007. 73:96–102.
15. Ritter M, Oechslin E, Sütsch G, Attenhofer C, Schneider J, Jenni R. Isolated noncompaction of the myocardium in adults. Mayo Clin Proc. 1997. 72:26–31.
16. Soler R, Rodriguez E, Monserrat L, Alvarez N. MRI of subendothelial perfusion deficits in isolated left ventricular noncompaction. J Comput Assist Tomogr. 2002. 26:373–375.
17. Oechslin EN, Attenhofer-Jost C, Rojas JR, Kaufmann PA, Jenni R. Long-term follow-up of 34 adults with isolated left ventricular noncompaction: a distinct cardiomyopathy with poor prognosis. J Am Coll Cardiol. 2000. 36:493–500.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download