Abstract
Background and Objectives
At birth, the fetal circulation must immediately adapt to extrauterine life. Our goal was to evaluate perinatal changes in the size of the aorta (Ao) and pulmonary artery (PA), and to investigate factors influencing these changes.
Subjects and Methods
Aortic and PA diameters were measured by echocardiography in 50 healthy term babies one day before and 4 to 5 days after birth.
Results
Compared with prenatal measurements, the Ao increased (from 7.4±0.6 mm to 8.4±0.6 mm, p<0.01) and the PA decreased (from 9.5±0.8 mm to 8.7±0.8 mm, p<0.01) in size after birth. The Ao/PA ratio increased from 0.78±0.07 before birth to 0.97±0.08 after birth (p<0.01), but there was no significant difference in the sum of the diameters of the great arteries between pre- and postnatal measurements. Postnatal increases in aortic size correlated negatively with prenatal aortic diameter (r=-0.37, p<0.05), but was not related to body weight. According to multiple regression analysis, significant variables for predicting perinatal changes in size of the Ao and PA were the prenatal Ao/PA ratio and the prenatal PA diameter, respectively.
Fetal circulation is unique in that the two ventricles function in parallel. At the moment of birth, fetal circulation must immediately adapt to extrauterine life. Physiologic changes in the circulation at birth affect ventricular size and the pulmonary circulation is separated from the systemic circulation with a dramatic increase in blood flow.1)
Previous studies on human fetal cardiac measurements by ultrasound techniques have provided quantitative data for assessing fetal cardiac growth.2)3) Sahn et al.3) also reported their results for measurements of fetal ventricles and great vessels plotting their measurements against fetal weight; however, their study had limited resolution with the use of lower frequency transducers, and serial measurements were not obtained for some of the study population.
In clinical practice, we have often experienced that a small aorta diagnosed prenatally by fetal echocardiography in a term baby becomes a nearly normal-sized aorta after birth. Thus, we undertook this study 1) to evaluate circulatory changes during the perinatal period by measuring the size of the aortic and pulmonary arteries before and after birth, and 2) to investigate factors influencing such perinatal changes.
Fifty term (37 to 40 weeks of gestation) pregnant women who planned to undergo Caesarean Section (C/S) delivery were prospectively enrolled. All mothers were healthy and none had a medical problem. None of them had any indications for fetal echocardiography but agreed to participate in this study and informed written consents were obtained. Using an Acuson 128XP (Mountain View, CA, USA) ultrasound system with a 5 MHz transducer, echocardiography was done for 50 normal babies one day before birth and again 4 to 5 days postnatally (all by a single examiner, HSK). No newborns had any structural anomalies including congenital heart disease. With the use of cine loop and zoom techniques, the internal diameters of the Ao and PA were measured during diastole when the semilunar valves were closed. The aortic diameter was measured at the annulus level in the long-axis view; the PA diameter was measured 1 mm above the sinotubular junction of the PA in the short axis view. Measurements were taken only when the image quality allowed clear definition of each structure with an insonation angle around 90°. The ratio of the aortic diameter to the PA diameter (Ao/PA), and the difference between prenatal and postnatal diameters, were calculated. After birth, physical parameters including body weight, head circumference and chest circumference were also recorded. To determine intraobserver variability, the diameters of the Ao and PA in 10 randomly selected newborns were measured by one observer (HSK) on two different occasions. For interobserver variability, they were examined by two observers (HSK, SS) who were unaware of the other's results.
Statistical analysis was performed with Statistical Package for Social Science (SPSS) 11.5 (SPSS Inc, Chicago, IL, USA). Differences in prenatal and postnatal measurements were compared using the paired t-test. Linear regression analysis was used to determine the relationship between great artery size and physical parameters. Factors influencing perinatal changes in size of the great arteries were investigated by multiple regression analysis. Variability was calculated as the difference from the mean of the two measurements and expressed as the percentage of the mean. A p<0.05 was considered significant.
Prenatally, the diameters of the Ao and PA were 7.4±0.6 mm and 9.5±0.8 mm, respectively. Postnatally, the aortic diameter increased to 8.4±0.6 mm and the PA diameter decreased to 8.7±0.8 mm (p<0.01) (Fig. 1). The Ao/PA ratio increased from 0.78±0.07 before birth to 0.97±0.08 after birth (p<0.01) (Fig. 2). However, there was no significant difference in the sum of the Ao and PA diameters before and after birth (16.9±1.2 mm vs. 17.1±1.3 mm, p=0.36) (Fig. 2).
The prenatal aortic diameter correlated positively with body weight (r=0.39, p<0.05), head circumference (r=0.28, p<0.05) and chest circumference (r=0.43, p<0.05), but the prenatal PA diameter did not correlate with these variables (not shown). Postnatal increases in aortic size correlated negatively with prenatal aortic diameter (r=-0.37, p<0.05) (Fig. 3A), but this change did not correlated with body weight (Fig. 3B). In contrast, postnatal decreases in PA size correlated with prenatal PA diameter (r=0.40, p<0.05) (Fig. 4A) and body weight (r=-0.42, p%0.05) (Fig. 4B). Multiple regression analysis indicated that significant predictors of perinatal changes in great artery size were the prenatal Ao/PA ratio (p=0.002) for an increase in aortic size, and prenatal PA diameter (p=0.01) for a decrease in the PA size.
Postnatal aortic diameter correlated with prenatal PA diameter (r=0.56, p<0.05), prenatal aortic diameter (r=0.67, p<0.05), and the sum of the prenatal Ao and prenatal PA diameters (r=0.71, p<0.05) (Fig. 5).
Intra-observer variability and inter-observer variability for the Ao were 2.7±2.1% and 2.9±2.1%, respectively; for the PA they were 3.1±2.2% and 5.3±2.6%, respectively. Intra-observer variability was smaller than inter-observer variability.
Several studies on fetal development of the great arteries have been reported,4)5) but there are few studies regarding perinatal changes in size of the fetal great arteries. To document perinatal changes, Sahn et al.3) examined 19 fetuses longitudinally beyond 20 weeks of gestation. In contrast, we examined term babies at 37 to 40 weeks of gestation and prenatal measurements were done one day before planned delivery (by C-section). Thus, our data may provide more accurate observations on circulatory changes that usually take place at birth.
In the fetus, the size of the great arteries increases with gestational age or fetal weight and the Ao is slightly smaller than the PA.3-5) In the studies of Shapiro et al.4) and Sharland and Allan,5) the Ao/PA ratio at 14 to 40 weeks of gestation was about 0.9 with a slight decrease during pregnancy, while in our study this ratio at 37 to 40 weeks of gestation was 0.78 and increased to 0.97 after birth. Our data suggest that the sum of the diameters of the great arteries remains constant (Fig. 2), although the Ao becomes larger and the PA becomes smaller. This results in an equalization of Ao and PA size after birth similar to postnatal changes in ventricular size.6-8) These findings support the idea that these two circulatory systems become separated at birth and handle the same amount of blood after birth.
It was interesting that only prenatal aortic diameter and not PA diameter was related to physical parameters. Therefore, one should consider not only gestational age but also body weight or other body parameters when the aorta is diagnosed as "small" by fetal echocardiography. In addition, postnatal increases in aortic size were significantly dependent on the prenatal Ao/PA ratio and was not related to body weight (Fig. 3B), suggesting that although there is a more significant difference in prenatal size between the Ao and PA in a smaller baby, a smaller Ao may become more enlarged in size after birth compared with the prenatal aortic size, even in a low-birth-weight infant as shown in Fig. 3A. In contrast, postnatal decreases in PA size were negatively related to body weight (Fig. 4B). Our data shows that the sum of the diameters of the great arteries stays constant. As such, the negative correlation between decreases in PA size and body weight may suggest that a smaller baby has a greater reduction in PA size than does larger babies, when they have a greater postnatal increase in aortic size, as mentioned above. To confirm these findings, there is a need for further clinical studies examining differences in postnatal changes of the great arteries according to body weight.
Postnatal aortic diameter had a robust positive correlation with prenatal Ao and PA diameters, and a stronger association with the sum of prenatal Ao and PA diameters (Fig. 5).
It is important to establish intra-observer variability and inter-observer to determine whether the changes in size of the great artery at birth are due merely to variability in measurements or to true changes. Mean intra- and inter-observer variability for aortic diameters ranged from 2 to 5%, which are acceptable levels. Practically, we determined only the variability in postnatal diameter of the Ao and PA and did not determine variability for prenatal Ao and PA diameters.
There are some limitations to our study. First, because of the narrow range of gestational ages and body sizes in the study population, our study showed small correlation coefficients between each variable. Second, considerable variation may exist in measuring techniques between the studies. We measured internal diameter of great arteries with exclusion of the thickness of the echo lines. Finally, the influence of the C-section itself can not be excluded as a contributor to physiologic changes at birth.
Despite these limitations, we conclude that fetal body weight should be considered in prenatally assessing aortic size. Although a greater difference exists prenatally between Ao and PA in lower-body-weight babies, circulatory changes at birth make the size of both arteries equal regardless of body weight.
Figures and Tables
Fig. 1
Postnatally, the diameter of the aorta (Ao) increases and that of the pulmonary artery (PA) decreases compared with measurements before birth.

Fig. 2
The Ao/PA diameter ratio increases from 0.78±0.07 before birth to 0.97±0.08 after birth (p<0.01), but there is no significant difference in the sum of the Ao and PA diameters before and after birth. Ao: aorta, PA: pulmonary artery.

Fig. 3
The postnatal increase in the diameter of the aorta (Ao) is related to the prenatal aortic diameter (A) and is not related to body weight (B).
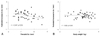
References
1. Hong JS, Choi JY, Zhu L, et al. Echocardiographic assessment of left ventricular diastolic function in transitional circulation period. Korean Circ J. 2006. 36:652–660.
2. Achiron R, Golan-Porat N, Gabbay U, et al. In utero ultrasonographic measurements of fetal aortic and pulmonary artery diameters during the first half of gestation. Ultrasound Obstet Gynecol. 1998. 11:180–184.
3. Sahn DJ, Lange LW, Allen HD, et al. Quantitative real-time corss-sectional echocardiography in the developing normal human fetus and newborn. Circulation. 1980. 62:588–597.
4. Shapiro I, Degani S, Leibovitz Z, Ohel G, Tal Y, Abinader EG. Fetal cardiac measurements derived by transvaginal and transabdominal cross-sectional echocardiography from 14 weeks of gestation to term. Ultrasound Obstet Gynecol. 1998. 12:404–418.
5. Sharland GK, Allan LD. Normal fetal cardiac measurements derived by cross-sectional echocardiography. Ultrasound Obstet Gynecol. 1992. 2:175–181.
6. Tan J, Silverman NH, Hoffman JI, Villegas M, Schmidt KG. Cardiac dimensions determined by cross-sectional echocardiography in the normal human fetus from 18 weeks to term. Am J Cardiol. 1992. 70:1459–1467.
7. Winsberg F. Echocardiography of the fetal and newborn heart. Invest Radiol. 1972. 7:152–158.
8. Wladimiroff JW, Vosters R, McGhie JS. Normal cardiac ventricular geometry and function during the last trimester of pregnancy and early neonatal period. Br J Obstet Gynaecol. 1982. 89:839–844.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


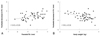

 XML Download
XML Download