Abstract
Posteroseptal accessory pathways are often associated with coronary sinus diverticula. These diverticula contain myocardial coats which serve as a bypass tract. We report a 54-year-old woman who underwent radiofrequency (RF) catheter ablation for Wolff-Parkinson-White (WPW) syndrome. The surface electrocardiography (ECG) demonstrated pre-excitation, indicating a posteroseptal accessory pathway. A catheter ablation via a transaortic approach failed to ablate the accessory pathway. Coronary sinus venography revealed the presence of a diverticulum near the ostium. An electrogram in the neck of the diverticulum showed the coronary sinus myocardial extension potential, which was successfully ablated by delivery of RF energy.
Radiofrequency (RF) catheter ablation has become the treatment of choice for supraventricular tachycardia (SVT) in patients with Wolff-Parkinson-White (WPW) syndrome.1)2) The posteroseptal and left posterior accessory pathways are sometimes located in the epicardial region and associated with ablation failure due to the complex anatomic arrangement.3) This kind of epicardial accessory pathway results from a connection between an extension of the coronary sinus (CS) myocardial coat along the middle cardiac vein, the posterior coronary vein, or the neck of a CS diverticulum and the left ventricular epicardium.4) A surface electrocardiography (ECG) is useful for predicting the epicardial location of a posteroseptal accessory pathway5) and CS angiography is often helpful for delineating the coronary venous anatomy. In the past, accessory pathways associated with CS diverticula were a significant cause of ablation failure. With mounting recognition of the importance of this anatomy, however, most of these accessory pathways have become readily ablated.
We report a case of an accessory pathway associated with a diverticulum inserting into the proximal coronary sinus, which was successfully ablated in the neck of the diverticulum.
A 54-year-old woman with WPW syndrome was referred for an electrophysiologic study. She had recurrent supraventricular tachycardia with symptoms of palpitations, dizziness, and diaphoresis. These episodes led her to curtailment of daily housework.
A surface ECG demonstrated ventricular pre-excitation with an isoelectric delta wave in V1 and a negative delta wave in leads II, III, and aVF, indicating a posteroseptal accessory pathway (Fig. 1). A transthoracic echocardiography revealed no structural heart disease.
An electrophysiologic study (EPS) was performed after the patient's consent to the procedure. Quadripolar catheters were introduced through the femoral vein and positioned in the right atrium (RA) and the right ventriclular apex (RVA). A hexapolar catheter was positioned in the His bundle area. A decapolar catheter was positioned in the CS via the left subclavian vein. At the time of the EPS, the delta wave was not present. RVA pacing produced an eccentric retrograde activation sequence and revealed the earliest atrial activation of the CS ostium with a ventriculoatrial interval of 132 ms. Programmed atrial pacing initiated orthodromic atrioventricular reciprocating tachycardia (AVRT). Mapping during AVRT showed an atrial activation sequence identical to that which occurred during RVA pacing. In tachycardia, introduction of ventricular extrastimuli 10-30 msec ahead of the His potential caused reproducible advancement of the next atrial sequence. A 7 French ablation catheter with a 4 mm tip electrode (EZ Steer™, Biosence Webster, Diamond Bar, CA, USA) was introduced via the femoral artery and placed under the mitral valve close to the annulus. Applications of RF energy (40W at 60℃) delivered in the posteroseptal region of the mitral annulus failed to eliminate the accessory pathway.
The ablation catheter was then placed into the CS via the femoral vein and mapping with RVA pacing was performed. However, repeated attempts of RF energy application to the earliest atrial activation site during RVA pacing failed to abolish the accessory pathway.
CS venography was performed and it demonstrated a diverticulum inserting into the proximal CS with a narrow neck (Fig. 2). The ablation catheter was repositioned in the neck of the diverticulum and recorded an early retrograde atrial signal representing CS activity (Fig. 3A). Ablation in this area under temperature control with a maximum pre-set energy of 15W achieved successful abolition of ventriculoatrial conduction via an accessory pathway (Fig. 3B).
Epicardial accessory pathways are most commonly found in the posteroseptal and left posterior regions. These accessory pathways are sometimes associated with CS anomalies, such as diverticula and fusiform or bulbous enlargement. The finding of a steep negative delta wave in lead II is known to be predictive of epicardial accessory pathways. It has been reported that the sensitivity of a negative delta wave in lead II in identifying a CS accessory pathway is >70%.4-6) The ECG of the present case showed a positive delta wave in lead I, an isoelectric delta wave in V1, and negative delta waves in leads II, III, and aVF. In addition, the R wave was greater in amplitude than the S wave in lead V1. The ECG algorithm5) to identify the location of an accessory pathway did not fit exactly in this case. Based on the ECG findings of a steep negative delta wave in the current case, it was necessary to predict the presence of an epicardial accessory pathway at the beginning of the procedure.
In the largest series to report the incidence of CS diverticula, of 480 patients with a posteroseptal or left posterior accessory pathway, Sun et al.4) demonstrated a CS diverticulum in 36 (7.5%). Based on this landmark clinical study,4) CS diverticula were shown to contain myocardial fibers which connect to both the ventricle and the CS myocardial coat. The connections between the CS myocardial coat and the ventricle could serve as an accessory pathway. In patients with a CS diverticulum, an accessory pathway potential is often recorded from the neck of the diverticulum. A characteristic activation pattern can be recorded from the mapping catheter placed in the coronary venous system during retrograde conduction over epicardial posteroseptal accessory pathways. The first potential is recorded from the CS diverticulum and is generated by the CS myocardial extension. The second potential is recorded along the floor of the CS and usually has leftward activation sequence because of the fiber orientation of the CS musculature. In the case described herein, we observed a very early potential at the neck of the CS and successfully ablated the accessory pathway.
Because of the close proximity of the CS ostium and posterolateral branch of the right coronary artery, caution should be exercised during application of RF energy into the CS. When the optimal ablation site is near the passage of the right coronary artery, saline-irrigated RF ablation or cryoablation is recommended to avoid a complication of coronary artery stenosis.
In summary, it is now well-appreciated that ablation of a posteroseptal accessory pathway needs a CS venography and careful evaluation of CS recordings for myocardial coat potentials. We have reported a patient who had WPW syndrome with a posteroseptal accessory pathway associated with a CS diverticulum. RF ablation in the neck of the CS effectively eliminates the accessory pathway conduction. The current case highlights the potential importance of contrast CS venography and identification of myocardial coat potentials in patients with a posteroseptal accessory pathway which is difficult to ablate by the endocardial approach.
Figures and Tables
Fig. 2
Coronary sinus venography showing a diverticulum with a narrow neck (arrows) near the ostium.
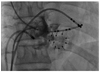
Fig. 3
Surface ECG and electrogram recordings during ventricular pacing. A: early retrograde sharp atrial potential representing coronary sinus activity (arrow). B: electrograms during ablation showing disconnection of the accessory pathway. ECG: electrocardiography, HRA: high right atrium, ABL: ablation, CS: coronary sinus, RVA: right ventricular apex, Stim: stimulation.

References
1. Jackman WM, Wang XZ, Friday KJ, et al. Catheter ablation of accessory atrioventricular pathways (Wolff-Parkinson-White syndrome) by radiofrequency current. N Engl J Med. 1991. 324:1605–1611.
2. Chen SA, Tsang WP, Hsia CP, et al. Radiofrequency catheter ablation for treatment of Wolff-Parkinson-White syndrome: short- and long-term follow-up. Int J Cardiol. 1992. 37:199–207.
3. Lee MH, Ahn S, Ku BK, et al. Catheter ablation of the posteroseptal accessory pathways. Korean Circ J. 1997. 27:407–416.
4. Sun Y, Arruda M, Otomo K, et al. Coronary sinus-ventricular accessory connections producing posteroseptal and left posterior accessory pathways: incidence and electrophysiological identification. Circulation. 2002. 106:1362–1367.
5. Arruda MS, McClelland JH, Wang X, et al. Development and validation of an ECG algorithm for identifying accessory pathway ablation site in Wolff-Parkinson-White syndrome. J Cardiovasc Electrophysiol. 1998. 9:2–12.
6. Takahashi A, Shah DC, Jais P, Hocini M, Clementy J, Haissaguerre M. Specific electrocardiographic features of manifest coronary vein posteroseptal accessory pathways. J Cardiovasc Electrophysiol. 1998. 9:1015–1025.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download