Abstract
Pulmonary thromboembolism is a very rare event in children, but the mortality rate is reported to be approximately 10%. The majority of children with thromboemboli have multiple risk factors, such as a catheter-related thrombosis, an infection, and a congenital prothrombotic disorder. Hypereosinophilia is very rarely associated with pulmonary emboli in adults; however, this condition has not been reported in children. We present a 12-year-old boy who had a pulmonary thromboembolism and deep vein thrombosis associated with hypereosinophilia and thrombocytopenia. The thromboembolism was managed with anticoagulant therapy and the hypereosinophilia resolved spontaneously.
Venous thromboembolism (VTE) is a term that encompasses deep vein thrombosis (DVT) and pulmonary embolism (PE). An evaluation of the National Hospital Discharge Survey and census date for VTE in the United States reported an annual incidence of 0.49 per 10,000, with peak rates in the neonatal period and adolescence.1)2) The majority of children with VTE have multiple risk factors for thromboembolic disease at presentation, such as catheter-related thrombosis, infection, and congenital prothrombotic disorders.3) A PE is a very rare event in children, but the mortality rate is reported to be approximately 10%.4) Hypereosinophilia is rarely associated with a PE in adults; however, this condition has not been reported in children. We present a case of a pulmonary thromboembolism and DVT associated with hypereosinophilia.
A 12-year-old boy was admitted to the hospital 10 days after the onset of cough, blood-tinged sputum, fever, right flank pain, and non-specific bilateral knee pain. Two weeks prior to admission, the patient went on a 10 hour automobile trip and ate raw fish. He showed symptoms and signs of pneumonia. On the laboratory examination, he had leukocytosis with eosinophilia (peak ratio, 35%), thrombocytopenia (minimum, 33,000/mm3), and an elevated C-reactive protein concentration (15.5 mg/dL). He was treated with antibiotics at a local hospital, but the symptoms did not remit. He was transferred to our institute for persistent symptoms and newly found signs of pulmonary hypertension on echocardiogram {trivial tricuspid valve regurgitation (TR) with a velocity of 3.2 m/sec}. On admission, he had a persistent cough and blood-tinged sputum. On physical examination, he had tachycardia (100 beats per minute), tachypnea (39 breaths per minute), and rales in the right lung field. He did not have cyanosis of the lips. The biochemical profile on admission was as follows: hemoglobin, 9.9 g/dL; white blood cell (WBC) count, 19,080/mm3 with 32.8% eosinophils; platelet count, 64,000/mm3; erythrocyte sedimentation rate, 6 mm/hr; and C-reactive protein, 9.2 mg/dL. A coagulation assay revealed a slightly prolonged prothrombin time and activated partial thromboplastin time and elevated fibrinogen (PT INR, 1.33; aPTT, 46.5 sec; and fibrinogen, 585 mg/dL). A chest X-ray demonstrated right lower lung field haziness (Fig. 1). An echocardiogram showed probable mild pulmonary hypertension with trivial TR (velocity, 3 m/sec), but there were no other abnormal findings. The myocardial thickness and both systolic and diastolic function of the ventricles were within normal limits. An electrocardiogram was unremarkable. A chest computerized tomography (CT) scan showed PE in the right upper and lower lobar pulmonary arteries and the left lower lobar pulmonary artery. A pulmonary infarction was demonstrated in the right lower lobe on the chest CT scan (Fig. 2A and B). A CT angiography showed a DVT in the left mid-femoral-to-popliteal and posterior tibial veins (Fig. 2C). A diffuse decrease in perfusion in the right lung and a focal decrease in perfusion in the superior segment of the left lower lung were confirmed by lung perfusion scan (right:left=40:60) (Fig. 3). Bone marrow aspiration showed high ratios of eosinophils to leukocytes with normal maturation. The marrow blasts were not increased. The patient showed no evidence of allergic disease and parasitic infection for the elevated eosinophil count in the peripheral blood and bone marrow. Other known causes of eosinophilia were not identified. These data led us to diagnose primary hypereosinophlia. However, there was no other end organ dysfunction which might be associated with hypereosinophlic syndrome. We also failed to find any other specific cause of hypercoagulation (antithrombin III activity, 91%; protein C, 75%; protein S, 82%; and homocysteine, 6.5 µmol/L). Antinuclear antibody, anticardiolipin antibody, and lupus anticoagulant levels were all either negative or within the normal range.
After diagnosis of a PE, he was initially treated with heparin (50 U/kg bolus followed by 17 U/kg/hr), then low-molecular-weight heparin (1 mg/kg/dose q 12 hours) for 1 month. The heparin was gradually switched to warfarin. The fever and cough subsided 10 days after admission. One week after admission, the platelet count dropped to 38,000/mm3.
On the 14th day after admission, the peripheral eosinophil count increased to 53.4% of the WBC (17,250/mm3; total eosinophil count, 9,210/µL). In 1 month, the eosinophilia and thrombocytopenia resolved spontaneously. His general condition gradually improved with the concomitant resolution of the pulmonary thromboembolism and pulmonary hypertension. The patient was discharged 1 month after admission on warfarin, which was adjusted to a PT INR of 2.0.
Eosinophilia was observed for a total of 2 months. Based on Doppler sonography, the thrombus in the lower extremity had resolved by 3 months. He took warfarin for 9 months and an anti-platelet medication (aspirin) for >1 year without any other complications. Fifteen months after admission, the peripheral blood examination revealed the following findings: hemoglobin, 14.8 g/dL; WBC count, 5,920/mm3 with 4.2% eosinophils; and platelet count, 230,000/mm3. While remaining on aspirin, he had no specific symptoms on evaluation in the outpatient clinic (Fig. 4).
A PE occurs when a segment of a thrombus within the deep venous system detaches from the vessel, goes to the lungs, and lodges in the pulmonary arteries. The pelvic and deep veins of the lower extremities are a common source of PE.5) In 1856, Rudolf Virchow identified predisposing thrombotic factors, which include blood stasis, endothelial injury of the vein, and alteration in blood coagulability. A VTE is a multifactorial disease involving both genetic and circumstantial risk factors. Mutations in the genes for anticoagulant proteins, such as antithrombin, protein C, and protein S are important risk factors. Factor V Leiden and prothrombin G20210A mutations are mild congenital thrombophilia traits. Circumstantial factors include increasing age, immobilization, surgery, pregnancy, oral contraceptives, hormone replacement, and inflammatory conditions.1)3)6) The thrombotic events occur when one or more of the circumstantial risk factors occur together. Genetic risk factors also predispose to a hypercoagulable state.
In this report, the patient had a high percentage of eosinophils at the onset of the thromboembolism. Hypercoagulation as a result of hypereosinophilia has previously been reported,7)8) but the mechanism remains poorly understood. Previous studies have demonstrated that eosinophils release toxic cationic proteins, which include eosinophil cationic protein, eosinophil-derived neurotoxin, major basic protein, eosinophil peroxidase, and platelet-activating factor.8) These granular proteins may promote platelet activation and coagulation, which inhibit the anticoagulation activity of thrombomodulin.7-9) In the patient presenting with peripheral blood eosinophilia, reactive causes should be investigated first, including parasitic infections, allergic disorders, malignancies, and collagen vascular disease. The patient in this report had a history of eating raw fish, but we did not find evidence of a parasitic infection; all the other conditions were excluded. If eosinophilia persists with an unknown etiology, the diagnosis of hypereosinophilic syndrome should be considered. Hypereosinophilic syndrome is defined as an eosinophil count >1,500/µL that is sustained for >6 months without any other clear cause.10) This patient had transient eosinophilia and recovered spontaneously, although the actual onset of eosinophilia was not identified. There may be other unidentified underlying causes for eosinophilia.
This patient had no typical symptoms and signs of a DVT. The symptoms, chest radiograph findings, and blood analysis mimicked a pneumonia. A PE may show non-specific signs and symptoms or no symptoms, although it can be fatal. Especially in the pediatric age group, the diagnosis of a PE may be delayed longer than in the adult age group. A chest CT is useful for diagnosis of a pulmonary thromboembolism. Pulmonary angiography has been the gold standard for diagnosing a PE, but it is more invasive and time-consuming than a CT. A lung perfusion scan and cardiac echocardiography are also helpful.
The treatment of a VTE in hemodynamically stable patients is anticoagulation, while thrombolytic therapy is indicated in massive iliofemoral DVT and PE with hemodynamic instability. The echocardiographic evidence of right ventricular dysfunction can be helpful in determining whether or not a patient needs thrombolytic therapy.11) The duration of therapy should be decided by causes and underlying conditions.
In conclusion, we have presented a case of a pulmonary thromboembolism which was accompanied by idiopathic hypereosinophila in a child.
Figures and Tables
Fig. 1
Chest radiograph on admission. Chest radiography showed right lower lung haziness mimicking lobar pneumonia.
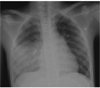
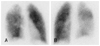
Fig. 2
Computed tomography with angiography on admission. A and B: contrast-enhanced CT angiography showed filling defects (arrows) in right upper lobar pulmonary artery (A) and both lower lobar pulmonary arteries (B) which represent pulmonary thromboembolism. There is a wedge-shaped peripheral consolidation with a central lucency in the right lower lobe which represents a pulmonary infarction. C: indirect CT venography showed a filling defect (arrow) in the left femoral vein which represents deep vein thrombosis.

References
1. Goldenberg NA, Bernard TJ. Venous thromboembolism in children. Pediatr Clin North Am. 2008. 55:305–322. vii
2. Stein PD, Kayali F, Olson RE. Incidence of venous thromboembolism in infants and children: data from the National Hospital Discharge Survey. J Pediatr. 2004. 145:563–565.
3. Biss TT, Brandao LR, Kahr WH, Chan AK, Williams S. Clinical features and outcome of pulmonary embolism in children. Br J Haematol. 2008. 142:808–818.
4. van Ommen CH, Heijboer H, Buller HR, Hirasing RA, Heijmans HS, Peters M. Venous thromboembolism in childhood: a prospective two-year registry in The Netherlands. J Pediatr. 2001. 139:676–681.
5. Browse NL, Thomas ML. Source of non-lethal pulmonary emboli. Lancet. 1974. 1:258–259.
6. Moon HJ, Rhim CY, Kim GW, et al. Risk factors of deep vein thrombosis and pulmonary embolism in Korean. Korean Circ J. 2005. 35:474–479.
7. Sato Y, Fukunaga T, Hayashi T, Asada Y. Hypereosinophilic syndrome associated with occlusive coronary thrombosis and right ventricular thrombus. Pathol Int. 2008. 58:138–141.
8. Uemura K, Nakajima M, Yamauchi N, Fukayama M, Yoshida K. Sudden death of a patient with primary hypereosinophilia, colon tumours, and pulmonary emboli. J Clin Pathol. 2004. 57:541–543.
9. Sherer Y, Salomon O, Livneh A, Pras M, Langevitz P. Thromboembolism in a patient with transient eosinophilia and thrombocytopenia. Clin Lab Haematol. 2000. 22:247–249.
10. Weller PF, Bubley GJ. The idiopathic hypereosinophilic syndrome. Blood. 1994. 83:2759–2779.
11. Ribeiro A, Lindmarker P, Juhlin-Dannfelt A, Johnsson H, Jorfeldt L. Echocardiography Doppler in pulmonary embolism: right ventricular dysfunction as a predictor of mortality rate. Am Heart J. 1997. 134:479–487.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download