Abstract
A 48-year-old woman visited the emergency department with shock due to a urinary tract infection. The patient, who had a history of hypertension and diabetes mellitus, presented with precordial ST-segment elevation and Q waves, along with an increase of cardiac enzymes. An echocardiography showed moderately reduced systolic function, severe apical left ventricular ballooning, and a dynamic left ventricular outflow tract obstruction with a pressure gradient of 109 mmHg. Coronary angiography demonstrated normal coronary arteries. At the 1-month echocardiographic follow-up, the apical ballooning and left ventricular systolic function had recovered completely. There was no residual left ventricular intra-cavity gradient at rest, but it was induced in low-dose dobutamine stress-echocardiography. We demonstrated that dynamic left midventricular obstruction in the setting of either increased catecholamine stress or hypovolemia could develop Tako-tsubo cardiomyopathy.
Tako-tsubo cardiomyopathy is characterized by the findings of acute onset, transient, left ventricular dysynergy (typically apical ballooning with concomitant compensatory hyperkinesis of the basal wall) with chest pain, electrocardiographic changes, and a minimal increase in myocardial enzymes, mimicking acute coronary syndrome in patients without significant stenosis on coronary angiography.1)
The pathophysiology of Tako-tsubo cardiomyopathy has not been fully clarified and we report a patient with Tako-tsubo syndrome in whom the clinical findings elucidated one of the mechanisms underlying this syndrome.
A 48-year-old woman was transferred to the emergency department in a stuporous mental status. She had been bedridden due to the sequalae of an intracranial hemorrhage and had an inability to complain of any symptoms. She had been having cold sweats for 3 days. She was currently being treated for diabetes mellitus and hypertension.
The initial blood pressure was 70/30 mmHg, the heart rate was 90 bpm, the respiratory rate was 22 bpm, and the oxygen saturation was normal. After infusion of intravenous dopamine at a rate of 30 µg/kg/min, the blood pressure increased to 110/60 mmHg. The body temperature was 38℃. On physical examination, the lung sounds were normal and the heart sounds revealed tachycardia with a grade 3/6 systolic murmur.
The electrocardiogram (ECG) showed ST-segment elevation with Q waves in V1-4, I, and aVL, which were not present in the ECG 3 months previously (Fig. 1). There was an increase in the following myocardial enzymes: troponin I (7.0 ng/mL; normal range, 0-0.7 ng/mL), troponin T (0.25 ng/mL; normal range, 0-0.1 ng/mL), and myoglobin (145 ng/mL; normal range, 0-11 ng/mL). The brain natriuretic peptide level was 3,122 pg/mL (normal range, 0-100 pg/mL). The C-reactive protein level was 30 mg/L (normal range, 0-8 mg/L), the white blood cell count was 9,820/µL and the neutrophil count was 6,874/µL. The renal profile revealed a creatinine of 1.7 mg/dL (normal range, 0.6-1.3 mg/dL) and a urea of 41 ng/mL (normal range, 5-23 ng/mL) with an increased sodium level of 161 mEq/L (normal range, 135-145 mEq/L). The fractional excretion of sodium was 0.1%. The urine analysis showed a leukocyte level of 10-19/hpf. The chest X-ray and brain CT showed no remarkable findings.
The baseline echocardiography showed mid and apical akinesia involving the anteroseptum and anterior wall of the left ventricle (LV) with moderately reduced systolic function and an ejection fraction of 32%. The echocardiogram also showed characteristic severe apical LV ballooning, hypercontractility of the basal segments, and a dynamic LV outflow tract obstruction with a pressure gradient of 109 mmHg (Fig. 2).
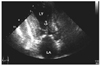
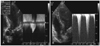
We suspected acute myocardial infarction manifesting as an elevation of the ST-segment and increased cardiac enzyme activity. Reperfusion therapy was not done because the onset of symptoms had occurred more than 12 hours earlier. Initially she was treated with aspirin, clopidogrel, subcutaneous low molecular weight heparin, a HMG-CoA reductase inhibitor, and intravenous quinolone for a urinary tract infection (UTI). The patient was admitted to the Cardiology Care Unit. After conservative treatment, including hydration and administration of antibiotics for the UTI, LV the apical ballooning and wall motion abnormality had partially improved on the follow-up echocardiography on day 5. However, the systolic anterior motion (SAM) at the level of the mitral chordae remained (Fig. 3). Coronary angiography demonstrated normal coronary arteries. An echocardiography on day 27 showed complete normalization of the apical wall motion abnormalities, apical ballooning, and LV systolic function. There was no residual LV intracavity pressure gradient at rest. On day 28, a dobutamine stress-echocardiography was performed to identify whether the LV intra-cavity pressure gradient was inducible. At the peak dose of dobutamine (40 µg/min/kg), a dynamic LV mid-cavity pressure gradient of 158 mmHg was induced and this gradient resolved during the recovery period (Fig. 4).
There are several existing hypotheses regarding the pathophysiology of Tako-tsubo cardiomyopathy. One of the hypotheses suggests that left ventricular outflow tract (LVOT) obstruction has a role. A sigmoid interventricular septum, a small LVOT, a reduced LV volume (primarily in women), and an abnormal orientation of a slack mitral apparatus are known to be geometrical predisposing factors for dynamic LVOT obstruction, effectively subdividing the LV into two functionally different chambers with a marked increase in wall stress in the high pressure apical chamber. The resulting structural arrangement would reduce sub-endocardial coronary flow in the apical chamber. Increased oxygen demand and reduced coronary perfusion pressure may combine to produce myocardial ischemia, myocardial stunning, regional wall motion abnormalities, and associated T-wave changes. Such is manifested in the setting of intense adrenergic stimulation or hypovolemia.2) With a combination of appropriate hydration and beta-blockade or a fall in catecholamine levels, the intraventricular gradient would be resolved and apical function would be recovered.2) Twelve of 72 patients (18%) in the Tsuchihashi series,3) and 2 of 13 patients as reported by Desmet6) showed a significant intraventricular gradient on an early echocardiogram. Merli et al.1) found transient intraventricular obstruction and a localized mid-ventricular septal thickening in four consecutive patients with Tako-tsubo cardiomyopathy.
A small body surface area (BSA) has also been postulated to be the cause of Tako-tsubo cardiomyopathy. Cocco et al.5) found that patients with stress-induced cardiomyopathy were of short stature, small BSA, and underdeveloped coronary arteries. An alternative hypothesis is that myocardial stunning is the underlying mechanism of this syndrome; however, there is no evidence for an obstructive or flow-limiting lesion on coronary angiography.3) Transient vasospasm of an epicardial coronary artery could be an etiology, although the area of akinesia does not correspond to the perfusion territory of a single coronary artery.1) Multiple coronary vasospasm could represent an another putative pathophysiologic mechanism, but vasospasm was provoked in no more than 21% of patients according to several reports.3) Enhanced sympathetic activity has also been hypothesized to have a crucial role. Catecholamine-mediated epicardial or microvascular vasoconstriction generates a base-to-apex perfusion gradient, which could result in regional differences in myocardial blood flow.1) Wittstein et al.4) demonstrated markedly higher plasma catecholamine levels at presentation among patients with stress-induced cardiomyopathy than among those with myocardial infarction.
In Korea, Lee et al.7) reported, for the first time, a single center prospective study of the clinical manifestations and the progress of stress-induced cardiomyopathy, but the precise etiology was left undecided.
We performed a dobutamine stress echocardiography after improvement in wall motion, which showed that the LV mid-cavity gradient was provoked at the peak dose. Based on our findings of this case, dynamic mid-cavity obstruction in the setting of either increased catecholamine stress or hypovolemia might be an important pathogenesis of Tako-tsubo cardiomyopathy. However, further studies are needed to confirm this because it remains possible that the observed intraventricular gradient might be a consequence rather than a cause of apical ballooning.
The clinical manifestations of stress-induced cardiomyopathy mimics acute coronary syndrome, so there is a risk of unnecessary and invasive medical diagnostics, such as coronary angiography and of the potentially dangerous fibrinolytic therapy. Physicians should be aware of and consider stress-induced cardiomyopathy in the differential diagnosis of acute coronary syndromes and acute cardiac failure.
Figures and Tables
Fig. 1
Change of electrocardiographic finding over the course. A: electrocardiography on admission, showing ST-segment elevation with Q waves in V1-V4, I, and aVL. B: electrocardiography 3 months before admission, showing left ventricular hypertrophy. C: electrocardiography on day 28, showing T wave inversion in V2-6.
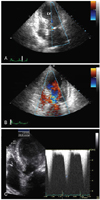
Fig. 2
Initial echocardiography on admission. A: end-systolic echocardiogram, showing left ventricular apical ballooning. B: end-diastolic echocardiogram, showing flow acceleration in the left ventricular outflow tract. C: continuous wave Doppler ultrasound showing a left ventricular outflow tract pressure gradient of 109 mmHg.
References
1. Merli E, Sutcliffe S, Gori M, Sutherland GG. Tako-tsubo cardiomyopathy: new insights into the possible underlying pathophysiology. Eur J Echocardiogr. 2006. 7:53–61.
2. Villareal RP, Achari A, Wilansky S, Wilson JM. Anteroapical stunning and left ventricular outflow tract obstruction. Mayo Clin Proc. 2001. 76:79–83.
3. Tsuchihashi K, Ueshima K, Uchida T, et al. Transient left ventricular apical ballooning without coronary artery stenosis: a novel heart syndrome mimicking acute myocardial infarction. J Am Coll Cardiol. 2001. 38:11–18.
4. Wittstein IS, Thiemann DR, Lima JA, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005. 352:539–548.
5. Cocco G, Chu D. Stress-induced cardiomyopathy: a review. Eur J Intern Med. 2007. 18:369–379.
6. Desmet WJ, Adriaenssens BF, Dens JA. Apical ballooning of the left ventricle: first series in white patients. Heart. 2003. 89:1027–1031.
7. Lee HH, Gwon HC, Kim BJ, et al. Clinical manifestation of novel stress-induced cardiomyopathy mimicking acute myocardial infarction: single center prospective registry. Korean Circ J. 2002. 32:1054–1063.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download