Abstract
Spontaneous retroperitoneal hemorrhage is a rare complication after percutaneous coronary intervention (PCI). The patient can be in danger if bleeding is not stopped immediately. However, it is not easy to control the bleeding completely because the bleeding foci can be multiple and there is a rich network of collateral circulation. We report a case of spontaneous retroperitoneal hemorrhage successfully treated using multiple microcoils. One year later, panhypopituitarism occurred as a likely consequence of the accompanying hypovolemic shock.
Spontaneous retroperitoneal hemorrhage occurs in a variety of clinical circumstances, including tumors, hemodialysis, polycythemia vera, percutaneous interventions, and anticoagulation treatment.1-4) Surgical treatment is indicated if the patient remains unstable despite adequate fluid and blood resuscitation. Recently, there have been many reports of successful treatment by endovascular intervention using a variety of techniques and devices, and this is now considered to be a better treatment option than surgery, when possible. A case report involving spontaneous retroperitoneal hemorrhage in a patient with myocardial infarction receiving intravenous heparin has been reported;5) however, the patient also received intravenous urokinase. We report a case of successful endovascular intervention using microcoils and gelform in a patient with spontaneous retroperitoneal hemorrhage after intravenous heparin treatment complicated by panhypopituitarism due to hypovolemic shock.
A 57-year-old female with a history of hypertension for 4 years presented to our hospital complaining of chest pain at rest. She did not have a history of bleeding and did not bruise easily. The physical examination on admission revealed the following vital signs: blood pressure, 110/70 mmHg; and heart rate, 70/min. Laboratory testing revealed a hemoglobin level of 10.6 g/dL; the platelet count, PT, and aPTT were all within normal limits. Coronary angiography revealed an 80% stenosis of the mid-left circumflex artery and a 50% stenosis of the proximal right coronary artery. We prescribed aspirin (200 mg) and clopidogrel (300 mg) as a loading dose and enoxaparin (6,000 IU subcutaneously twice a day). Two days later, we successfully deployed a paclitaxel-eluting stent (Taxus™; Boston Scientific, Natick, MA, USA) at the left circumflex lesion using a right femoral approach, with unfractionated heparin (5,000 IU intravenously) just before the percutaneous coronary intervention (PCI). Several hours later, the patient complained of right lower quadrant abdominal pain and we could see that the abdomen was distended with tenderness. The patient's vital signs revealed that the blood pressure had decreased to 70/40 mmHg and the heart rate had increased to 100/min. The laboratory findings showed that the hemoglobin level had dropped to 7.3 g/dL. An abdominopelvic CT scan showed a huge retroperitoneal hemorrhage, including an active bleeding focus (Fig. 1). An urgent angiography was performed and revealed that the right access site was not remarkable, but two active bleeding foci, branches of the right lumbar artery and the right circumflex iliac artery, were visualized (Fig. 2). All bleeding foci, including collateral vessels, were successfully embolized using 7 microcoils (Fig. 3) and 5 pints of packed red blood cell were transfused. However, the blood pressure did not increase above 90/60 mmHg and the heart rate was 110/min. The repeat hemoglobin level was 8.1 g/dL. Fortunately, the patient's urine output was preserved and the creatinine remained within normal limits. A follow-up CT scan 4 days after the embolization showed a larger hemorrhage with another bleeding focus (Fig. 4A) along with newly developed hydronephrosis in the right kidney due to ureteral compression (Fig. 4B). An additional angiography revealed multiple bleeding foci from branches of the right ovarian artery (Fig. 5A). Due to the tortuosity of the right ovarian artery, we could not advance the guide wire, so we decided to use gelform and successfully occluded the right ovarian artery at the ostial portion (Fig. 5B). The patient complained of chest tightness with shortness of breath and a chest radiograph demonstrated a pleural effusion in both lungs. A chest CT scan and diagnostic thoracentesis showed a bilateral hemothorax (Fig. 6A), so we inserted a chest tube into the right pleural space. A total of 350 cc of blood was initially drained. After this intervention, the patient's vital signs stabilized and no bleeding foci were found in the follow-up abdominopelvic CT scan. Both hemothoraces also improved (Fig. 6B). Seven days later, we were able remove the chest tube. Before discharge, we confirmed that there was no evidence of platelet or coagulation disorders. Blood tests were as follows: fibrinogen, 350 mg/dL; protein C activity, 94%; protein C antigen, 72.4%; protein S antigen, 40.6%; von Willebrand factor activity, 243%; factor assay VIII, 112%; factor assay V, 96%; antinuclear antibody, (-); anti-double stranded deoxyribonucleic acid (ds DNA) antibody, (-); and antiplatelet antibody (-). One month after the initial CT scan, a follow-up CT scan showed that the hemorrhage had increased slightly in size (Fig. 7A) and was more mature. Furthermore, the hydronephrosis had progressed with a deterioration in renal function (Fig. 7B). However, the 3 month follow-up CT scan revealed a much smaller volume of hemorrhage (Fig. 8A) and an improvement in the hydronephrosis with a corresponding improvement in renal function (Fig. 8B). The hemorrhage was almost completely resolved by the 7 month follow-up (Fig. 9) and there was no evidence of infection. One year later, the patient presented to our hospital complaining of anorexia and loss of appetite. The patient's serum sodium and potassium levels were 117 mEq/L and 3.3 mEq/L, respectively. The laboratory findings were consistent with panhypopituitarism; specifically, endocrine profile was as follows: prolactin, 3.7 ng/mL; growth hormone, 0.15 ng/mL; luteinizing hormone (LH), 2.01 mIU/mL; follicle stimulating hormone (FSH), 7.24 mIU/mL; estradiol, 5.02 pg/mL; T3, 0.195 ng/mL; free T4, 0.179 ng/dL; thyroid stimulation hormone (TSH), 1.95 µIU/mL; cortosol at 8 am, 4.0 µg/dL; and adrenocorticotropin hormone (ACTH), 13.48 pg/mL. The sella MRI showed an empty sella (Fig. 10). The patient reco vered after once daily doses of oral prednisolone (5 mg) and levothyroxine (0.05 mg).
Access site bleeding is a common minor complication after PCI and it can usually be controlled easily with manual compression. However, retroperitoneal hemorrhage from the puncture site can be lethal because it is difficult to stop bleeding by external compression. The worldwide incidence of retroperitoneal hemorrhage following cardiac catheterization is 0.15%.6) The clinical presentation is varied and may be subtle, therefore early detection can be delayed. There are often no obvious stigmata of an underlying expanding hematoma or cutaneous bruising. Retroperitoneal hemorrhage should be suspected in patients with significant flank pain, groin pain, or relative hypotension and tachycardia that transiently improve with fluid resuscitation, and a fall in hemoglobin level following a procedure. Most hemodynamically stable patients can be managed with fluid resuscitation, correction of coagulopathy, and blood transfusion. But if the patient is unstable, conservative management should be avoided.7) In the past, open surgical repair has been considered the only option in uncontrollable hemodynamic collapse,8) but removal of the hematoma may increase rebleeding by releasing the tamponade effect.9) Recently, many reports have discussed successful endovascular interventions using microcoils, gelform, or stent grafts. Microcoils are probably the most widely used and safest form of treatment, but it should be remembered that proximal coiling of the bleeding artery may not be sufficient when there is a rich network of collateral arteries and new arteries may develop after embolization of the bleeding artery.10) It is important to place coils both proximally and distally to the bleeding site to prevent rebleeding. In the patient presented herein, we used seven microcoils in the bleeding site, including all collaterals.
Retroperitoneal hemorrhage can occur spontaneously in the absence of an obvious underlying pathology or trauma. It is most commonly seen in patients who have been anticoagulated, have bleeding abnormalities, or are receiving hemodialysis. Several sporadic cases have been reported, but the exact pathologic mechanism is not yet known. One possible hypothesis is diffuse occult vasculopathy and arteriosclerosis of the small vessels in the retroperitoneum.11) A second suggested hypothesis is heparin-induced immune micriangiopathy.12) It can be assumed that our patient was initially exposed to unfractionated heparin by heparin mixed saline during the diagnostic coronary angiography, in which case antibodies to heparin were produced, so that when the patient was exposed to heparin again during PCI, there was an antigen-antibody reaction, resulting in microvascular damage. The final possible hypothesis is that an unrecognized minor trauma in the microcirculation during physical activity and vomiting or coughing may have induced sustained bleeding in the absence of clotting factors.13)
An abdominal compartment syndrome is a rare complication that acutely increases intra-abdominal pressure by massive retroperitoneal hemorrhage, which can cause a decrease in cardiac output, anuria, or worsening of renal failure, intestinal ischemia, and respiratory failure.14) In our case, hydronephrosis and acute renal failure developed due to an extrinsic compression of the retroperitoneal hemorrhage. However, renal function gradually improved as the size of the hematoma decreased. Therefore, if the acute bleeding is controlled and vital signs are stabilized, we think it is a wise choice to wait and monitor the patient's condition despite the enormous size of the hematoma, as in the case presented. In our patient, panhypopituitarism developed one year after the initial bleeding, which is the first report of empty sella caused by acute spontaneous retroperitoneal bleeding.
In conclusion, although our case featured an extremely large retroperitoneal hemorrhage, we successfully stopped the acute bleeding through endovascular intervention and eventually the mass spontaneously resolved without surgical intervention.
Figures and Tables
Fig. 1
Initial abdominopevic CT scan. A huge hematoma in the retroperitoneal space was found, and there was contrast enhancement leakage inside of the mass (arrow) implying active bleeding. CT: computed tomography.
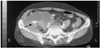
Fig. 2
Initial finding of the angiography. The urgently performed angiography showed two active bleeding foci (arrows): branches of the right circumflex iliac artery (A) and right lumbar artery (B).

Fig. 3
Coil embolization. A total of 7 microcoils were used to eliminate all bleeding foci, including all collateral arteries. After coil embolization, a final angiography was performed and comfirmed that there was no contrast agent leakage.
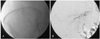
Fig. 4
Follow-up chest CT scan 4 days after the initial embolization. A: the size of the hematoma increased, but no suspicious bleeding sites were found. B: a hydronephrosis in the right kidney developed, probably caused by a right ureteral compression by the mass. CT: computed tomography.

Fig. 5
Another bleeding focus. A: the angiography revealed multiple bleeding foci (dark arrows) from branches of the right ovarian artery. B: after gelfoam injection, angiography showed a totally occluded right ovarian artery at the ostium portion (dotted arrow).
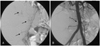
Fig. 6
Both hemothoraces. A: a chest CT scan revealed bilateral hemothoraces, confirmed by diagnostic thoracentesis. A chest tube insertion was performed. B: 9 days later, a follow-up chest CT scan showed a much improved hemothorax. CT: computed tomography.
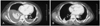
Fig. 7
One month after the initial chest CT scan. A: the size of the hematoma slightly increased without any bleeding points and the wall of the hematoma was more densely enhanced, indicating that the hematoma had matured. B: the hydroneprosis of the right kidney became more aggravated. CT: computed tomography.
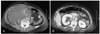
Fig. 8
Three month follow-up chest CT scan. A: the size of the hematoma was decreased. B: the hydronephrosis of the right kidney had nearly resolved. CT: computed tomography.
References
1. Tappe U, Kristen F, Löffler A, Keller HW. Spontaneous retroperitoneal hematoma in adrenal metastasis. Dtsch Med Wochenschr. 1997. 122:471–474.
2. Malik A, Capling R, Bastani B. Enoxaparin-associated retroperitoneal bleeding in two patients with renal insufficiency. Pharmacotherapy. 2005. 25:769–772.
3. Ishihara S, Yasuhara H, Ogawa S, Muto T. Successful surgical treatment for spontaneous retroperitoneal hematoma in polycythemia vera: report of a case. Surg Today. 2000. 30:199–201.
4. Jeong TK, Jeong GH, Park BS, et al. Dalteparin sodium-associated retroperitoneal hematoma in a patient with diabetic nephropathy. Korean J Med. 2003. 64:322–327.
5. Kim HJ, Kim DY, Whang MG, Jo HK. A case of spontaneous retroperitoneal hemorrhage due to iliopsoas muscle hematoma in patient with myocardial infarction receiving intravenous heparin. Korean Circ J. 1998. 28:1798–1801.
6. Sreeram S, Lumsden AB, Miller JS, Salam AA, Dodson TF, Smith RB. Retroperitoneal hematoma following femoral arterial catheterization: a serious and often fatal complication. Am Surg. 1993. 59:94–98.
7. Chan YC, Morales JP, Reidy JF, Taylor PR. Management of spontaneous and iatrogenic retroperitoneal hemorrhage: conservative management, endovascular intervention or open surgery? Int J Clin Pract. 2007. [Epub ahead of print].
8. Baker BH, Baker MS. Indications for exporing the retroperitoneal space. South Med J. 1980. 73:969–970.
9. Grimm MR, Vrahas MS, Thromas KA. Pressure-volume characteristics of the intact and disrupted pelvic retroperitoneum. J Trauma. 1998. 44:454–459.
10. Kauppila LI. Blood supply of the lower thoracic and lumbosacral regions: postmorterm aortography in 38 young adults. Acta Radiol. 1994. 35:541–544.
11. Torres GM, Cernigliaro JG, Abbitt PL, et al. Iliopsoas compartment: normal anatomy and pathologic processes. Radiographics. 1995. 15:1285–1297.
12. McCort JJ. Intraperitoneal and retroperitoneal hemorrhage. Radiol Clin North Am. 1976. 14:391–405.
13. Berna JD, Zuazu I, Madrigal M, et al. Conservative treatment of large rectus sheath hematoma in patients undergoing anticoagulant therapy. Abdom Imaging. 2000. 25:230–234.
14. Dabney A, Bastani B. Enoxaparin-associated severe retroperitoneal bleeding and abdominal compartment syndrome: a report of two cases. Intensive Care Med. 2001. 27:1954–1957.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download