Abstract
Background and Objectives
Stanford type A aortic dissection is a potentially catastrophic event that requires surgical repair, on an emergency basis. The extent of arch repair that should be carried out during emergency surgery of this type is controversial. This study was designed to evaluate the results of arch replacement carried out during acute type A dissection.
Subjects and Methods
28 patients with Stanford type A dissection and who underwent arch replacement between 1995 and 2006 were reviewed.
Results
Hospital mortality was 3.6% (1 patient), and transient neurocognitive dysfunction was observed in 5 patients. During the follow-up period (mean 26±20 months; range 1 to 66 months), 3 patients underwent reoperation due to descending thoracic or abdominal aortic aneurysm. There was no late death. Follow up computed tomography was performed in 15 patients and false lumen disappeared totally or partially in 10 patients (66.7%).
Stanford type A acute aortic dissection is a medical condition that requires emergent surgery.1) The surgical objectives are to recover the continuity of the aortic wall and to reduce the acute phase deaths due to cardiac tamponade, aortic regurgitation, and brain injury. The technical procedures primarily consist of ascending aorta replacement; however, conflicting opinions exist about the optimal extent of aortic correction. In the past 10 years, significant advances in diagnosis, surgical methods, the pre-operative work-up, the post-operative management, and the methods of cardiopulmonary bypass have been made. A patent false lumen post-operatively has been reported to be one of the risk factors that affects long-term treatment outcomes. In association with this, as one of the efforts which has been made to obliterate a false lumen, aortic arch replacement is of increasing interest. In the current study, we examined the risk factors and mid-term follow-up results in patients who underwent aortic arch replacement at our medical institution.
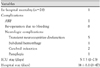
Of 230 patients who underwent surgery for acute aortic dissection between November 1995 and July 2006, 28 patients (12.2%) who underwent aortic arch replacement were enrolled in the current retrospective analysis of medical records. Patients who underwent replacement of the ascending aorta or hemi-arch replacement and those who had chronic aortic dissection or combined aortic aneurysm were excluded from the current analysis. The mean age was 54±12 years (range, 30-77 years) and the male-to-female ratio was 14:14. In all patients with the exception of one, the surgery was performed at the time of admission in an emergency setting. The elapsed time between hospital arrival and onset of surgery was 2.8±2.7 hour (range, 0.4-10.8 hour). With the exception of one patient who required an aortic arch replacement because of distal anastomosis site bleeding after hemi-arch replacement and in whom the intimal tear was present in the ascending aorta, all the patients had intimal tears detected in the aortic arch (Table 1).
All the surgical procedures were performed via a median sternotomy. Through a right subclavicular incision, an 8-mm polytetrafluorethylene (PTFE) graft was anastomosed to the right subclavian artery. Then, the arterial cannula was inserted through the 8-mm graft. Under moderate hypothermic circulatory arrest, an open distal anastomosis of the aortic arch was performed. In all the patients with the exception of three, clamping of the innominate artery and a direct cannulation of the left common carotid artery and left subclavian artery with antegrade selective cerebral perfusion was performed. The circulatory arrest time was 32.5±18.7 minute and the selective cerebral perfusion time was 69.7±34.2 minute. In recent years, cerebral oximetry was monitored to prevent ischemic brain injury and proximal anastomosis was performed during cooling (Table 2).
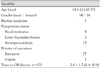
The methods of anastomosing the aortic arch branches included 11 cases of connecting single artificial blood vessels, 9 cases of 2 artificial blood vessels, 7 cases of 3 artificial blood vessels, and 1 case of 4 artificial blood vessels (Fig. 1).
The Bentall operation was performed in three cases and an aortic valve sparing root replacement was performed in one case. Other concomitant procedures included two cases of coronary artery bypass grafting and two cases of femoro-femoral bypass.
Post-operatively, there was one early death case (3.6%). This patient arrived at our department 24 hours after a diagnosis of acute aortic dissection was made. At that time, malperfusion of the lower extremities was present. This patient was treated with replacement of the aortic arch and femoro-femoral bypass surgery. However, due to post-operative multi-organ failure, including acute renal failure, the patient expired.
Post-operative complications included eight cases of re-operation due to bleeding and central nervous system (CNS) complications, including five cases of transient cognitive impairment, two cases of cerebral infarction, one case of subarachnoid hemorrhage, and one case of paraplegia due to a spinal infarction which occurred on post-operative day 3. Excluding one case of paraplegia, all the neurologic complications improved by the time of discharge (Table 3). The median length of hospital stay was 14 days (range, 8-47 days). All patients discharged were follow-up until recently. In these patients, the mean follow-up period was 26±20 months (range, 1-66 months). There were no late deaths. A follow-up CT angiography was performed in 15 patients. A false lumen was completely lost or left in the abdominal aorta in only 10 cases (66.7%). During the follow-up period, three patients needed surgical treatment of the descending thoracic aorta or abdominal aorta due to expansion of the descending aorta with a patent false lumen in post-operative months 3, 11, and 27. All these patients survived (Table 4).
Stanford type A acute aortic dissection is a medical condition in which the initial 48-hour mortality reaches 50% after the onset of dissection, thus requiring emergency surgical treatment. Kim et al.2) reported the characteristics of aortic dissection in Korean patients. These authors noted that aortic dissection mainly occurred in patients between 60 and 69 years of age, and in 75% of these patients, the dissection of the ascending aorta was concurrently present and the major cause was hypertension.2) The major objectives of the surgical procedure for this medical entity are to reduce the deaths due to cardiac tamponade, acute aortic valve dysfunction, and coronary artery involvement that can be caused by dissection or CNS complications. It is well-established that replacement of the ascending aorta must be considered first. However, at present, in accordance with the location of the intimal tear, the extent of dissection and the pathologic status of the aortic wall at which the dissection occurred, conflicting opinions exist regarding the optimal extent of distal resection.3)
With the recent advancement in the diagnostic technique for acute aortic dissection, the pre-operative work-up, post-operative management, and the methods of cardiopulmonary bypass, attempts have been made to enhance the early surgical results as well as to reduce the incidence of follow-up complications.4-6) It has been reported that the occurrence of secondary aneurysms due to a patent false lumen is the risk factor affecting the long-term treatment outcomes, so the distal extent of resection should include the intimal tear and could produce good treatment outcomes.7-9)
In an autopsy study, Roberts et al.10) reported that the dissection would progress in the antegrade direction in approximately 1/3 of cases, the retrograde direction in 1/3 of cases, and in both directions in 1/3 of the cases in which the intimal tear occurred in the aortic arch. These authors emphasized the importance of active inspection of the aortic arch to find the initimal tear site and the correction of the tear in cases in which the intimal tear was absent in the ascending aorta.10)
In cases in which the intimal tear was present in the lesser curvature of the aortic arch of the opposite part of the innominate artery, a hemi-arch replacement could be used for the correction. However, controversies exist regarding the extent of distal resection in cases in which it is located more distally because it might increase the surgical risks.11)12) The rate of early mortality following surgery of acute type A aortic dissection varies, and has been reported to be 9% to 25%. In recent years, aortic arch replacement in acute type A aortic dissection has shown good long-term treatment outcomes without increasing surgical mortality significantly, so it should be actively considered.13)14)
In the current study, of 28 patients, there was only 1 case (3.6%) of early death following the surgery. The corresponding patient visited us 24 hours after a diagnosis of acute aortic dissection was made with malperfusion of the lower extremities. Total replacement of the aortic arch and femoro-femoral bypass surgery were performed. Postoperatively, for the treatment of compartment syndrome in the lower extremities, a fasciectomy was performed. However, the patient presented with multi-organ dysfunction, including acute renal failure, from which the patient died on post-operative day 13. The death was regarded to be due to non-surgical causes. As described above, replacement of the aortic arch could be performed with a low rate of early death.
The post-operative complications included eight reoperations due to bleeding; the CNS complications included five cases of transient cognitive impairment, two cases of cerebral infarction, one case of subarchnoid hemorrhage, and one case of paraplegia due to spinal infarction, which occurred on post-operative day 3. Excluding one case of paraplegia, all the patients had an improvement in symptoms at the time of discharge. Pacini et al.15) reported that risk factors for developing transient cognitive impairment in the surgical treatment of the aortic arch include old age, and surgeries which are concomitantly performed with coronary bypass surgery or aortic valve replacement. These authors also noted that elderly patients are particularly vulnerable to brain injury due to systemic inflammation associated with cardiopulmonary bypass or intra- or post-operative low cardiac output. Furthermore, they reported that transient cognitive impairment developed in 7.2% of the cases in which selective cerebral perfusion occurred.15) In the current study, post-operative transient cognitive impairment developed in 5 cases (17%) and this figure was higher than other reports. However, these cases mostly developed in the early years. Following the implementation of antegrade selective brain reperfusion and monitoring with a cerebral oximeter, it has rarely developed. Also, it is assumed that the neurologic deficits which did not occur prior to the surgery would be expressed following the surgery. Of the neurologic deficits, two patients were 60 years of age or older, but there was no statistical significance regarding the occurrence of transient cognitive dysfunction. Also, in two patients in whom coronary bypass surgery was concomitantly performed, transient cognitive impairment did not occur. According to Park et al.16) there was no significant difference in the transient cognitive impairment between antegrade and retrograde selective cerebral reperfusion, although the former shortened the recovery time.16) In the current study, with the exception of three patients, all the remaining patients underwent antegrade selective cerebral reperfusion. Whether the recovery time was shortened after using antegrade selective cerebral perfusion could not be compared. However, all five consecutive patients in whom antegrade selective cerebral perfusion was recently performed, recovery was achieved in the early period.
Paraplegia developed in 1 case in which the intraoperative distal ischemic time was 52 minutes and the rectal temperature was 18℃. Patients diagnosed with alcoholic cardiomyopathy recovered with no notable problems on post-operative day 1. On post-operative day 3, paraplegia abruptly developed at the level of T10. On aortic angiography, there was no significant difference in the pressure between a false and a true lumen. There was no reperfusion derangement in the intercostal arteries. Based on these findings, spinal infarction occurred because of thromboembolism. This led to the administration of anti-coagulation, but a recovery could not be achieved.
Re-operation due to bleeding was performed relatively frequently, most often in the early years. In recent years, with the advances in surgical techniques and the use of biological glues, it has rarely been performed.
As noted earlier, in cases in which a false lumen was patent following surgery, secondary complications, such as aortic aneurysm or peripheral ischemia, may occur and the death occurs due to the rupture of aortic aneurysm. In the initial surgery, attempts must be made to reduce the rate of occurrence of patent false lumens. During the follow-up period, if the aortic size is increased, a second procedure should be performed in a timely manner to reduce the occurrence of late deaths. In the current study, to reduce the rate of patent false lumens, resection of the aortic arch included the site of the intimal tear. The methods of anastomosing the arch branches were 11 cases of a connection of a single artificial graft, 9 cases of 2 artificial grafts, 7 cases of 3 artificial grafts, and 1 case of 4 artificial grafts. Follow-up CT angiography was performed in 15 cases in which the dissection was present up to the descending aorta before surgery. Of the cases, a false lumen was completely lost or patent in the abdominal aorta in only 10 cases (66.7%). This confirmed that the replacement of the aortic arch is an effective surgical modality in reducing the residual false lumen. During the follow-up period, redo-surgery was performed in 3 patients (11.1%). A partial loss of the proximal false lumen was observed, but the diameter of the distal thoracic aorta was increased in two cases. In one case, while the false lumen was patent, the diameter of the abdominal aorta was increased. Post-operatively, there were no deaths. A recovery was achieved without any notable complications. Following surgery of an acute type A aortic dissection, redo-surgery was most often due to the development of an aortic aneurysm. Halstead et al.17) reported that the incidence of aortic aneurysms was relatively higher in the descending thoracic aorta. Yeh et al.18) reported that the incidence of a patent false lumen was significantly lower in a group of patients in whom the replacement of the aortic arch was performed, including the site of the intimal tear, so they insisted that replacement of the aortic arch should be performed actively to prevent aortic enlargement following the initial surgery. Choi et al.19) compared the CT findings between the group in which the medical treatment was made following the aortic dissection and the group in which the surgical treatment was performed following the onset of aortic dissection. These authors reported that a proportion of the true lumen was increased and the sectional area of the total aorta was decreased on follow-up CT scans in the group in which the intimal tear was corrected with the surgical treatment.19) It can also be inferred that redo-aortic surgery would reveal many technical difficulties if it includes the aortic arch and it would be performed easily in cases in which the aortic arch was replaced at the initial surgery, so the replacement of the aortic arch should be performed for the first surgery.
Figures and Tables
Fig. 1
Methods of anastomosis for arch vessels. Arch and arch vessels were anastomosed with single graft in 11 cases, 2 grafts in 9 cases, 3 grafts in 7 cases, and 4 grafts in 1 case.

References
1. Szeto WY, Gleason TG. Operative management of ascending aortic dissections. Semin Thorac Cardiovasc Surg. 2005. 17:247–255.
2. Kim KS, Ahn JK, Kim JH, Lim HK, Lee BH, Lee CK. Characteristics of aortic dissection in Korea. Korean Circ J. 1987. 17:743–760.
3. Suehiro K, Pritzwald-Stegmann P, West T, Kerr AR, Haydock DA. Surgery for acute type a aortic dissection a 37-year experience in Green Lane Hospital. Heart Lung Circ. 2006. 15:105–112.
4. Ando M, Takamoto S, Okita Y, Morota T, Matsukawa R, Kitamura S. Elephant trunk procedure for surgical treatment of aortic dissection. Ann Thorac Surg. 1998. 66:82–87.
5. Mizuno T, Toyama M, Tabuchi N, Wu H, Sunamori M. Stented elephant trunk procedure combined with ascending aorta and arch replacement for acute type A aortic dissection. Eur J Cardiothorac Surg. 2002. 22:504–509.
6. Kazui T, Yamashita K, Washiyama N, et al. Aortic arch replacement using selective cerebral perfusion. Ann Thorac Surg. 2007. 83:S796–S798.
7. Fattori R, Bacchi-Reggiani L, Bertaccini P, et al. Evolution of aortic dissection after surgical repair. Am J Cardiol. 2000. 86:868–872.
8. Bernard Y, Zimmermann H, Chocron S, et al. False lumen patency as a predictor of late outcome in aortic dissection. Am J Cardiol. 2001. 87:1378–1382.
9. Cho KJ, Woo JS, Bang JH, Choi PJ. Comparison of the mid-term changes at the remnant distal aorta after aortic arch replacement or ascending aortic replacement for treating type A aortic dissection. Korean J Thorac Cardiovasc Surg. 2007. 40:414–419.
10. Roberts CS, Roberts WC. Aortic dissection with the entrance tear in transverse aorta: analysis of 12 autopsy patients. Ann Thorac Surg. 1990. 50:762–766.
11. Kazui T, Yamashita K, Washiyama N, et al. Impact of an aggressive surgical approach on surgical outcome in type A aortic dissection. Ann Thorac Surg. 2002. 74:S1844–S1847.
12. Watanuki H, Ogino H, Minatoya K, et al. Is emergency total arch replacement with a modified elephant trunk technique justified for acute type A aortic dissection? Ann Thorac Surg. 2007. 84:1585–1591.
13. Kazui T, Washiyama N, Muhammad BA, et al. Extended total arch replacement for acute type a aortic dissection: experience with seventy patients. J Thorac Cardiovasc Surg. 2000. 119:558–565.
14. Ochiai Y, Imoto Y, Sakamoto M, et al. Long-term effectiveness of total arch replacement for type A aortic dissection. Ann Thorac Surg. 2005. 80:1297–1302.
15. Pacini D, Di Bartolomeo R, Di Eusanio M, Pierangeli A. Antegrade selective cerebral perfusion during operations on the thoracic aorta: our experience. Ann Thorac Surg. 2000. 70:10–16.
16. Park I, Kim KT, Lee JT, Chang BH, Cho JY, Lee EB. Analysis of neurological complications on antegrade versus retrograde cerebral perfusion in the surgical treatment of aortic dissection. Korean J Thorac Cardiovasc Surg. 2005. 38:489–495.
17. Halstead JC, Meier M, Etz C, et al. The fate of the distal aorta after repair of acute type A aortic dissection. J Thorac Cardiovasc Surg. 2007. 133:127–135.
18. Yeh CH, Chen MC, Wu YC, Wang YC, Chu JJ, Lin PJ. Risk factors for descending aortic aneurysm formation in medium-term follow-up of patients with type A aortic dissection. Chest. 2003. 124:989–995.
19. Choi JO, Kim YS, Hwang ES, et al. Changes of aortic morphology after aortic dissection evaluated by CT angiography. Korean Circ J. 2002. 32:53–60.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download