Abstract
Pheochromocytomas presents with variable clinical manifestations. Cardiomyopathy caused by a pheochromocytoma is well known. We report the case of a 62-year-old woman with recurrent left ventricular dysfunction, who was subsequently found to have a pheochromocytoma. The patient had two different patterns of cardiomyopathy. Patients with a cardiomyopathy, of non-specific origin, should have a pheochromocytoma ruled out.
Pheochromocytomas are rare neuroendocrine tumors that secrete high levels of catecholamines and usually cause paroxysmal or sustained hypertension. Various forms of myocardial damage have also been reported with pheochromocytomas including cardiomyopathy. We report a patient who was treated for recurrent transient left ventricular (LV) dysfunction, with the final diagnosis of a catecholamine-induced cardiomyopathy.
In November of 1999, a 62-year-old woman with a history of diabetes mellitus and hypothyroidism, was admitted to the hospital with right renal cell carcinoma. The initial blood pressure was 120/70 mmHg. The electrocardiogram (ECG) showed normal sinus rhythm. The initial echocardiography showed normal regional wall motion and normal left ventricular systolic function. There was a 5×4 cm solid mass at the lower pole of the right kidney on the abdominal CT scan, suggesting a renal cell carcinoma. Angioembolization of the right renal cell carcinoma was performed on the 25th of November 1999. A right nephrectomy was planned for the following day. The preoperative blood pressure was 160/100 mmHg in the operating room, and the blood pressure increased to 230/110 mmHg after induction of anesthesia. The ECG changes included T wave inversions and ST segment depression in leads V1-6, which were new ECG findings. The operation was canceled.
Echocardiography was performed immediately and showed impaired wall motion of all basal and mid-ventricular segments of the left ventricle (Fig. 1). The creatine kinase-MB (CK-MB) level was elevated to 66.4 ng/mL by the next day. The ST-T abnormality was normalized in 24 hours. The Echocardiography was repeated after two weeks and showed normalization of the left ventricular wall motion. The coronary angiography showed normal coronary arteries on the 11th of December 1999 procedure. The renal cell carcinoma was treated with interferon from December 1999 to March 2000. The size of the right renal cell carcinoma was markedly reduced after the interferon treatment. Follow-up abdominal CT scanning was performed in April and November of 2000 (Fig. 2). A left adrenal mass was identified. The size of the mass was about 2 cm. The right renal cell carcinoma and the left adrenal mass were mildly enlarged by March 2002. The size of the left adrenal mass was about 2.5 cm (Fig. 2).
The patient had no subjective symptoms and was lost to follow up. In December 2003, the patient visited the emergency room (ER) with dizziness. The initial blood pressure was 150/70 mmHg. T-wave inversions were noted on the inferior ECG leads. Echocardiography showed impaired wall motion of the apical and mid-segments of the left ventricle and severely decreased LV systolic function (Fig. 3). The peak level of cardiac troponin I was 3.1 ng/mL. Technetium-99m methoxyiso-butylisonitrile (Tc-99m MIBI) single emission computerized tomography (SPECT) revealed no evidence of inducible ischemia. Echocardiography was repeated after one week, the LV systolic function and regional wall motion abnormalities had fully recovered. In September 2004, the left adrenal mass of the patient was enlarged (Fig. 2) and she was diagnosed with a pheochromocytoma. The 24 hour urine metanephrine was elevated at 3.478 mg/day, and the iodine-131 meta-iodobenzylguanidine (I-131 MIBG) adrenal scan was compatible with a pheochromocytoma (Fig. 4). The patient had a successful resection of the left adrenal tumor and right kidney in December 2004. Microscopic examination confirmed that left adrenal tumor was a pheochromocytoma and the right renal tumor was a renal cell carcinoma.
The left ventricular dysfunction syndrome is well known. However, the underlying pathophysiology of this condition remains unclear. Catecholamine-induced myocardial stunning is the best known and most widely accepted explanation. Catecholamine excess leads to cyclic adenosine mono-phosphate (AMP)-mediated calcium overload of the cell, with the resultant decrease in synthetic activity and viability.1)
Pheochromocytomas are derived from endocromaffin cells; they are catecholamine-secreting neuroendocrine tumors. Cardiomyopathy related to a pheochromocytoma is well known; associated dilated cardiomyopathy has been reported.4)5) In addition, many cases of Takotsubo cardiomyopathy have been reported.6)7) Recent case reports have described patients with lesions involving areas other than the apex.8-10) There appears to be no unique ventricular dysfunction pattern in catecholamine-induced cardiomyopathies.
In this case, the left adrenal pheochromocytoma might have been too small to identify on the initial CT scan. However, the fluctuating blood pressure (BP) in this patient and the growth of the left adrenal mass on the follow-up CT scan, suggested a relationship between the first event of LV dysfunction and the pheochromocytoma. This case demonstrates recurrent and variable manifestations of a catecholamine-induced cardiomyopathy in a patient with a pheochromocytoma. The cardiomyopathy was the inverted Takotsubotype for the first event, and later a conventional type with the presentation of apical ballooning. In this patient there were two different types of recurrent left ventricular dysfunction. The cardiomyopathy induced by catecholamine's or stress, can be completely reversed and potentially cured with the appropriate treatment. Therefore, early diagnosis and treatment is very important. The diagnosis of a pheochromocytoma should be considered in patients with left ventricular dysfunction and no other obvious diagnosis.3) If the imaging studies are negative, biochemical studies for pheochromocytoma should be performed. Especially in cases with recurrent left ventricular dysfunction.
Figures and Tables
Fig. 1
Echocardiography showing akinesis of the basal and mid-ventricular segments, with preserved contractility of the apical segments. A: apical 4 chamber view of end-diastole (Left) and end-systole (Right). B: apical 2 chamber view of end-diastole (Left) and end-systole (Right).
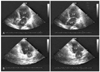
Fig. 2
An enhanced abdominal computed tomographic scan. A: November. 14, 2000-2 cm left adrenal mass with central low density lesion (arrow). B: March 8, 2002-the left adrenal mass increased to 2.5 cm (arrow). C: September 3, 2004-The size of left adrenal mass was about 3 cm (arrow).
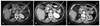
Fig. 3
Echocardiography performed in December of 2003 showing decreased motion of the apical and mid-segments, with preserved contractility of the basal segments. A: apical 4 chamber view of end-diastole (Left) and end-systole (Right). B: apical 2 chamber view of end-diastole (Left) and end-systole (Right). LA: left atrium, LV: left ventricular.
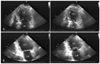
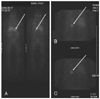
Fig. 4
I-131 MIBG scan performed 48 hours after isotope injection, shows increased tracer uptake in the left adrenal gland (arrow), and normal tracer activity in the liver and gastrointestinal tract (A and B). I-131 MIBG scan performed 72 hours after isotope injection, also shows increased uptake in the left adrenal gland (arrow, C). MIBG: metaiodobenzylguanidine.
References
1. Mann DL, Kent RL, Parsons B, Cooper G 4th. Adrenergic effects on the biology of the adult mammalian cardiocyte. Circulation. 1992. 85:790–804.
2. Szakacs JE, Cannon A. L-Norepinephrine myocarditis. Am J Clin Pathol. 1958. 30:425–434.
3. Sardesai SH, Mourant AJ, Sivathandon Y, Farrow R, Gibbons DO. Phaeochromocytoma and catecholamine induced cardiomyopathy presenting as heart failure. Br Heart J. 1990. 63:234–237.
4. Attar MN, Moulik PK, Salem GD, Rose EL, Khaleeli AA. Phaeochromocytoma presenting as dilated cardiomyopathy. Int J Clin Pract. 2003. 57:547–548.
5. Hong SK, Choi H, Lee SC. Reversal of dilated cardiomyopathy with medical therapy in a case of pheochromocytoma. Korean Circ J. 1998. 28:284–290.
6. Takizawa M, Kobayakawa N, Uozumi H, et al. A case of transient left ventricular ballooning with pheochromocytoma, supporting pathogenetic role of catecholamines in stress-induced cardiomyopathy or takotsubo cardiomyopathy. Int J Cardiol. 2007. 114:e15–e17.
7. Takeno Y, Eno S, Hondo T, Matsuda K, Zushi N. Pheochromocytoma with reversal of tako-tsubo-like transient left ventricular dysfunction: a case report. J Cardiol. 2004. 43:281–287.
8. Sanchez-Recalde A, Costero O, Oliver JM, Iborra C, Ruiz E, Sobrino JA. Images in cardiovascular medicine: pheochromocytoma-related cardiomyopathy: inverted Takotsubo contractile pattern. Circulation. 2006. 113:e738–e739.
9. van de Walle SO, Gevaert SA, Gheeraert PJ, De Pauw M, Gillebert TC. Transient stress-induced cardiomyopathy with an "inverted takotsubo" contractile pattern. Mayo Clin Proc. 2006. 81:1499–1502.
10. Copetti R, Gonano C, Colombo T, Cattarossi L. "Inverted Takotsubo" pattern. Resuscitation. 2007. 74:394.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download