Abstract
An unusual type of hypertrophic cardiomyopathy was diagnosed in a 17-year-old girl who presented with dyspnea on exertion. The hypertrophied myocardium was localized to the anterior portion of the left ventricle from the base to the apex without left ventricular outflow tract obstruction. On cardiac magnetic resonance imaging (MRI), patchy and linear delayed hyperenhancement was shown in the anterior and inferior mid-wall, which is not concordant
with the coronary artery territory.
Hypertrophic cardiomyopathy is a very heterogenous disease entity. Ventricular basal septal hypertrophy is by far the most common type of asymmetrical hypertrophy, with midventricular, apical, right ventricular, and rarer types of asymmetrical hypertrophy being far less common.1-3) We present a case of an uncommon type of hypertrophy diagnosed by echocardiography and cardiac MRI.
A 17-year-old girl was admitted for evaluation of dyspnea on exertion during 5 months. She had no history of diabetes, hypertension, or family members with sudden cardiac death. A 12-lead electrocardiogram (ECG) showed a sinus rhythm with ST segment elevation in leads III/aVF/aVR, ST depression in I/aVL/V5-6, and T inversion in leads V1-4 (Fig. 1).
Transthoracic echocardiography showed a significant asymmetric hypertrophy of the basal anterior wall which extended to the level of the apex. The maximal wall thickness was 21 mm and this hypertrophy was localized to the anterior wall (Fig. 2); the echogenecity was similar to other areas of the myocardium. There was no turbulent flow through the left ventricular outflow tract (LVOT) and the maximal pressure gradient was <30 mmHg at rest. Although the systolic function, as measured by the ejection fraction was within the normal range, deformation analysis using 2-dimensional strain echocardiography, which can detect early functional abnormalities, showed decreased circumferential and radial strain (Fig. 3). The patient was referred for cardiac MRI for further evaluation of myocardium.
On consecutive static images of short axis cines {steady-state free precession (SSFP)} in diastole, asymmetric hypertrophy of the anterior wall extended progressively from the base to the apex with a maximal thickness of 25 mm, which had an isointense signal compared with the surrounding myocardium (Fig. 4). The systolic motion of that part was normal and perfusion was good at the full thickness. Delayed contrast-enhanced MRI showed patch and linear hyperenhancement in the anterior and inferior mid-wall, which is not concordant with the coronary artery territory (Fig. 5). Based on the diagnosis of an unusual type of hypertrophic cardiomyopathy, she was treated with beta-blockers and she has been well with minimal symptoms and without any adverse cardiac events.
Hypertrophic cardiomyopathy (HCM) is a primary genetic heart disorder caused by sarcomere mutations and may account for up to 60% of unexplained left ventricular hypertrophy, making HCM the most common genetic cardiovascular disorder.4)5) The histopathologic hallmarks of HCM are myocyte hypertrophy with disarray and increased cardiac fibrosis; the phenotype of HCM is variable and involves myocardial thickening and obstruction of the ventricular outflow tract.6) Clinical progression can be indolent or more rapidly result in refractory symptoms and heart failure. So, it is very important to identify preclinical individuals and to treat with targeting preventive therapy.
Echocardiography had been used to diagnose HCM, evaluate the myocardial function, and predict the prognosis. Nagueh et al.7) described the decreased systolic, early diastolic, and late diastolic tissue Doppler velocities in HCM patients, and predicted HCM in subclinical patients using tissue Doppler images.8) Two-dimensional strain images can also reliably analyze myocardial deformation. Circumferential and radial strains decreased in all myocardial segments in the patients with HCM compared with physiologic hypertrophy.9) In the patient presented herein, only involved myocardial segments decreased circumferential and radial strain, showing normal tissue Doppler velocities and normal strain values in the other myocardium. Therefore, this case may be an early change of progressive HCM or a benign subgroup of unusual HCM.
The significance of MRI on HCM has been much more investigated over the past 20 years. The microvascular dysfunction by first-pass stress perfusion MRI images and fibrotic lesions in the myocardium by delayed contrast hyperenhancement (DCE) are useful to predict ventricular arrhythmia and sudden cardiac death. The extent of hyperenhancement is associated with progressive ventricular dilation, cardiac function, and markers of sudden death.10)11) Therefore, another component of the cardiac MRI examination may have an important role to evaluate patients with HCM, not only by increased myocardial wall thickness.12)
In the patient reported herein, the asymmetrical hypertrophy was limited to the anterior wall extending from the base to the apex; this type of HCM is the first case. The systolic strain decreased in the thickened myocardium; however, the delayed hyperenhancement on MRI was not massive, localized, and spotty. Follow-up examinations will be needed to evaluate disease progression and prognosis.
Figures and Tables
Fig. 1
Twelve-lead ECG showing sinus rhythm with ST segment elevation in leads III/aVF/aVR, ST depression in I/aVL/V5-6, and T inversion in leads V1-4. ECG: electrocardiogram.
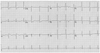
Fig. 2
Parasternal short axis view of transthoracic echocardiography. Hypertrophic myocardium was (white arrows) localized to the anterior wall from base (A), mid-level (B), to the apex (C).
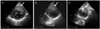
Fig. 3
Two-dimensional strain analysis. Each color represents each mid-LV segment as described in the left side of the images. The hypertrophied anterior segment, expressed as sky blue, decreased systolic deformation (strain) compared with other segments. A: circumferential strain. B: radial strain. LV: left ventricle.

Fig. 4
Consecutive static images of short axis cines {steady-state free precession (SSFP)} in diastole (A-F). Asymmetric hypertrophy of the anterior wall extended progressively from the base (A) to the apex (F) and iso-signal intensity of hypertrophied tissue compared with surrounding myocardium was seen.

References
1. Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA. 2002. 287:1308–1320.
2. Wigle ED, Rakowski H, Kimball BP, Williams WG. Hypertrophic cardiomyopathy: clinical spectrum and treatment. Circulation. 1995. 92:1680–1692.
3. Jeong JW. Hypertrophic cardiomyopathy. Korean Circ J. 2002. 32:7–14.
4. Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, Bild DE. Prevalence of hypertrophic cardiomyopathy in a general population of young adults: echocardiographic analysis of 4111 subjects in the CARDIA study. Circulation. 1995. 92:785–789.
5. Richard P, Charron P, Carrier L, et al. Hypertrophic cardiomyopathy: distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. Circulation. 2003. 107:2227–2232.
6. Klues HG, Schiffers A, Maron BJ. Phenotypic spectrum and patterns of left ventricular hypertrophy in hypertrophic cardiomyopathy. J Am Coll Cardiol. 1995. 26:1699–1708.
7. Nagueh SF, Bachinski LL, Meyer D, et al. Tissue Doppler imaging consistently detects myocardial abnormalities in patients with hypertrophic cardiomyopathy and provides a novel means for an early diagnosis before and independently of hypertrophy. Circulation. 2001. 104:128–130.
8. Nagueh SF, McFalls J, Meyer D, et al. Tissue Doppler imaging predicts the development of hypertrophic cardiomyopathy in subjects with subclinical disease. Circulation. 2003. 108:395–398.
9. Richand V, Lafitte S, Reant P, et al. An ultrasound speckle tracking (two-dimensional strain) analysis of myocardial deformation in professional soccer players compared with healthy subjects and hypertrophic cardiomyopathy. Am J Cardiol. 2007. 100:128–132.
10. Teraoka K, Hirano M, Ookubo H, et al. Delayed contrast enhancement of MRI in hypertrophic cardiomyopathy. Magn Reson Imaging. 2004. 22:155–161.
11. Moon JC, McKenna WJ, McCrohon JA, Elliott PM, Smith GC, Pennell DJ. Toward clinical risk assessment in hypertrophic cardiomyopathy with gadolinium cardiovascular magnetic resonance. J Am Coll Cardiol. 2003. 41:1561–1567.
12. Reichek N, Gupta D. Hypertrophic cardiomyopathy: cardiac magnetic resonance imaging changes the paradigm. J Am Coll Cardiol. 2008. 52:567–568.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download