Introduction
Ischemic heart disease is the leading cause of death worldwide. Recently, cell therapy for the treatment of heart failure has received considerable attention after new discoveries on the potential of adult stem cells showed an ability to differentiate into functional cardiomyocytes.1-4) However, the plasticity of bone marrow cells has been challenged by alternative mechanisms, such as cell fusion and paracrine action, but not transdifferentiation.5)6) Questions and controversies with regard to the mechanisms of myocardial regeneration still exist. In this context, studies are needed to elucidate the molecular mechanisms underlying cardiomyogenic differentiation of adult stem cells, as well as embryonic stem cells, in order to make progress toward the clinical application of cell therapy in patients with cardiovascular diseases.
The P19 embryonal carcinoma stem cell line has been widely used as a model system for the study of molecular mechanisms underlying cardiomyogenic differentiation.7-9) These cells can be maintained in an undifferentiated state in a monolayer without a feeder-cell layer. Such conditions allow for easy introduction of ectopic genes and the performance of experiments requiring a large quantity of cells.9)10) Cardiomyogenic differentiation of P19 cells has generally been induced by embryoid body (EB) formation in the presence of 0.5-1% dimethyl sulfoxide (DMSO) in bacterial dishes.7) We previously showed that 5-azacytidine could induce cardiac differentiation of P19 cells under confluent monolayer culture conditions without prior EB formation and DMSO exposure.9) To date, however, the differentiation efficacy of P19 cells to develop into the cardiomyogenic lineage remains low. Therefore, in this study we examined the effects of different culture conditions, including different concentrations of serum, 5-azacytidine, and culture time, on cardiomyogenic differentiation of P19 cells. The goal was to develop an efficient protocol for directing cardiomyogenic differentiation of P19 cells.
Materials and Methods
Culture and cardiac differentiation of P19 cells
The P19 cells were obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA). The cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco-BRL, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS, Gibco-BRL), 100 units of penicillin/mL, and 100 µg of streptomycin/mL. To induce cardiac differentiation, EB formation was induced by plating P19 cells on 10-cm bacterial dishes with 1×106 cells in 10 mL of DMEM supplemented with 1% DMSO (Sigma, St. Louis, MO, USA), 10% FBS, 100 units of penicillin/mL, and 100 µg of streptomycin/mL for 96 hours. The formed EBs were transferred to 24-well plates, and cultured in DMEM with 2% or 10% FBS for an additional 10 or 15 consecutive days in the presence of 0, 1, and 3 µM of 5-azacytidine (Sigma). The morphologic changes of the P19 cells were examined under an inverted microscope (Nikon, Tokyo, Japan) equipped with phase-contrast objectives and a digital camera.
Real-time polymerase chain reaction
Total ribonucleic acid (RNA) was extracted from P19 cells with Trizol reagent (Invitrogen, Carlsbad, CA, USA). Then, 0.5 µg of the total RNAs was treated with DNase (Promega, Madison, WI, USA) to remove the contaminated genomic deoxyribonucleic acid (DNA). The first-strand complementary DNA (cDNA) was synthesized from 0.5 µg of DNase-treated total RNA using 0.5 µg random hexamers, and 200 U Moloney murine leukemia virus reverse transcriptase (Invitrogen) at 37℃ for 60 minutes in a volume of 20 µL. The first strand cDNA (1 µL) was used for polymerase chain reaction (PCR) amplification in a 25 µL reaction mixture. Real-time PCR was performed using an iQ™ Cycler (Bio-Rad, Hercules, CA, USA); each reaction contained 25 µL of the iQ™ SYBER Green Supermix (Bio-Rad), 3 µL of forward primer (5 µM), 3 µL of reverse primer (5 µM), 5 µL of a 1 : 20 dilution of a cDNA, and 14 µL of H2O. The PCR conditions included a denaturation step (95℃ for 3 minutes), amplification and quantification repeated 45 times (94℃ for 15 seconds, 60℃ for 30 seconds, and 72℃ for 30 seconds), and melting curve analysis (55-95℃ with a heating rate of 0.05℃/second). The primers used for real-time PCR were as follows: GATA4 (CCTGCGGCCTCTACATGA, AGGGTCTCACCAGCAGGA, 136 bp); alpha-cardiac muscle actin (α-actin; GGAGAAG AGCTATGAACTTCCTGA, GCCAGCAGATTCCA TACCA, 112 bp); alpha-cardiac myosin heavy chain (α-MHC; GGATTCTCTGAAAAGTTAACCAGAGT, GGCGTTCCTTCTCTGACTTTC, 108 bp); cardiac muscle troponin T (cTnT; GGCTCACTTCGAGAAC AGGA, TCATTGCGAATACGCTGCT, 108 bp); and GAPDH (TTCACCACCATGGAGAAGGC, GGCAT GGACTGTGGTCATGA, 237 bp). For the selection of primers for real-time PCR, we designed intron spanning primers following the Roche Universal Probe Library method (www.roche-applied-science.com) to avoid genomic DNA amplification. We then selected primers that generated a single band of PCR product with the expected size by performing a standard reverse transcriptase-PCR (RT-PCR) (data not shown). The final primers were selected based on the linearity of the threshold cycle (Ct) values obtained in the serial dilutions of the template, with the negative controls containing no template.
The measurement of gene expression was assayed in triplicate. The relative gene expression levels were quantified based on the Ct, and normalized to the reference gene GAPDH.
Immunocytochemistry
For the immunostaining procedure, P19 cells were plated at a density of 1×106 cells on 10-cm bacterial dishes for 96 hours in the presence of 1% DMSO. The formed EBs were transferred onto 0.1% gelatin-coated coverslips in 12-well dishes, and cultured in DMEM with 10% FBS for an additional 10 days in the presence of 1 µM of 5-azacytidine. The formed EBs were then fixed during 20 minutes of incubation in PBS containing 4% paraformaldehyde, and then rinsed in PBS containing 0.1% Tween 20 (PBT) three times. The fixed cells were permeabilized for 30 minutes in PBS containing 0.5% Triton X-100, and then blocked with 10% normal goat serum in PBT for 1 hour. The cells were then incubated with sarcomeric α-actinin, cardiac myosin heavy chain, cardiac myosin light chain, and cTnT (all from Sigma) at 4℃ overnight in 2% normal goat serum in PBT. After washing in PBT, the cells were then stained with secondary Alexa Fluor488-conjugated anti-rat IgG (Molecular Probes, Eugene, OR, USA) for 30 minutes at room temperature, and washed three times in PBT. For the control experiments, the cells were stained with secondary antibodies only. The nuclei were stained with 4'6'-diamidino-2-phenylindole (DAPI), and the cells were mounted with fluorescent mounting medium (Dako, Carpinteria, CA, USA).
The fluorescence images were obtained using the TEFM Epi-Fluorescence system attached to an Olympus inverted microscope (Olympus, Tokyo, Japan).
Statistical analysis
The statistical analyses were performed using a t-test or the Student-Newman-Keuls' multiple comparison test. Statistical significance was set a priori at a p<0.05. All statistical values are expressed as the mean±standard deviation (SD). All statistical analyses were performed using SigmaStat3.1 software (SPSS Inc., Chicago, IL, USA).
Results
Effects of culture time on cardiomyogenic differentiation of the P19 cells
We examined the effects of culture time on cardiomyogenic differentiation of the P19 cells by SYBR Green-based quantitative real-time PCR assays with primers for GATA4, α-actin, α-MHC, and cTnT as cardiac-specific markers. The P19 cells were maintained in a monolayer of DMEM+10% FBS before induction of cardiac differentiation (Fig. 1A). To induce cardiac differentiation, they were cultured in a suspension in bacterial dishes for 96 hours in DMEM+10% FBS containing 1% DMSO to form EBs (Fig. 1B); the EBs were further cultured for 10 or 15 consecutive days in DMEM+10% FBS. Expression of GATA4, α-actin, α-MHC, and cTnT mRNA cardiac-specific markers was -1.97, -4.93, -33.54, and -15.92-fold higher, respectively, in 20-day cultures of P19 cells than 15-day cultures of P19 cells (Fig. 2). This result shows that cardiomyogenic differentiation of P19 cells increases as the culture time is extended.
Effects of serum concentration on cardiomyogenic differentiation of the P19 cells
We also analyzed the effect of serum concentrations on cardiomyogenic differentiation of the P19 cells by quantitative real-time PCR assay. The P19 cells were cultured in suspension in bacterial dishes for 96 hours in DMEM+10% FBS containing 1% DMSO to form EBs; the EBs were further cultured for 10 or 15 consecutive days in DMEM containing 2% or 10% FBS. The expression of GATA4, α-actin, α-MHC, and cTnT mRNA in the EBs cultured in DMEM containing 10% FBS were -7.15, -1.62, -8.35, and -6.75-fold higher, respectively, than the EBs cultured in DMEM containing 2% FBS for 10 consecutive days after EB formation (Fig. 3A). Furthermore, the expression of GATA4, α-actin, α-MHC, and cTnT mRNA in the EBs cultured in DMEM containing 10% FBS were also -1.84, -3.14, -3.42, and -2.41-fold higher, respectively, than the EBs cultured in DMEM containing 2% FBS for 15 consecutive days after EB formation (Fig. 3B). This result demonstrates that cardiomyogenic differentiation of the P19 cells was enhanced under high-serum culture conditions (10% FBS) compared to low-serum culture conditions (2% FBS), regardless of the culture time.
Effects of 5-azacytidine on cardiomyogenic differentiation of the P19 cells under different serum concentrations
Several studies have reported that 5-azacytidine, a DNA hypomethylating agent, stimulates the cardiac differentiation of stem cells isolated from various tissues, such as mesenchymal stem cells,11) human embryonic cells,12) cardiac stem cells,13) and P19 cells.9) Based on previous reports, we investigated the effects of 5-azacytidine on cardiomyogenic differentiation of the P19 cells by quantitative real-time PCR assay. The P19 cells were cultured in suspension in bacterial dishes for 96 hours in DMEM+10% FBS containing 1% DMSO to form EBs; the EBs were further cultured for 10 consecutive days in DMEM containing 2% (Fig. 4A) or 10% FBS (Fig. 4B). The expression of cardiac-specific markers, such as GATA4, α-MHC, and cTnT mRNA was highest in the cells treated with 1 µM 5-azacytidine, regardless of the serum concentrations (Fig. 4). In addition, the cells treated with 3 µM 5-azacytidine showed a statistically significant increase in the expression of cardiac-specific markers compared to the control cells (Fig. 4). This result demonstrates that cardiomyogenic differentiation of the P19 cells was the most effective with 1 µM of 5-azacytidine, regardless of the serum concentrations. In addition, stimulation by 5-azacytidine for cardiomyogenic differentiation was more distinct under low-serum culture conditions (2% FBS) compared to high-serum culture conditions (10% FBS).
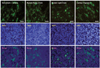
To further confirm the cardiomyogenic differentiation of the P19 cells at the protein level, we performed immunostaining with antibodies against cardiac-specific markers, including sarcomeric α-actinin, cardiac myosin heavy chain, cardiac myosin light chain, and cTnT after induction of cardiomyogenic differentiation of the P19 cells. The P19 cells were plated at a density of 1×106 cells on 10-cm bacterial dishes for 96 hours in the presence of 1% DMSO. The formed EBs were transferred onto 0.1% gelatin-coated coverslips in 12-well dishes, and cultured in DMEM with 10% FBS for an additional 10 consecutive days in the presence of 1 µM 5-azacytidine. A number of cells showing specific staining patterns for cardiac-specific markers were observed in the cardiomyocytes differentiated from the P19 cells (Fig. 5).
Discussion
Cardiomyocyte differentiation of stem cells in vitro is known to be affected by various environmental factors, such as the incubation period, medium composition, serum components, growth factors, and chemicals. Therefore, in this study, we investigated the effects of different concentrations of serum and 5-azacytidine, and culture time on the cardiomyogenic differentiation of P19 cells. We showed that cardiomyogenic differentiation of the P19 cells was enhanced in parallel with the length of the culture period. This result was in agreement with our previous findings that showed that the expression of cardiac-specific markers, such as GATA4, Nkx2.5, and cTnT after treatment with 1 µM 5-azacytidine in a P19 cell monolayer culture was up-regulated in a time-dependent manner.9)
In the present study, we compared the effects of serum concentrations on cardiac differentiation of the P19 cells. We showed that cardiomyogenic differentiation of the P19 cells was enhanced by high FBS (10%)-containing medium compared to low FBS (2%)-containing medium, regardless of the culture time period. Investigation of the stimulatory or inhibitory effects of FBS concentration on the cardiac differentiation of stem cells has been previously reported. Medium containing 20% FBS has been shown to promote more rapid down-regulation of the pluripotency marker, Oct 4, and increased expression of endodermal and mesodermal genes at the time of EB formation of H1 human embryonic stem cells (ESCs), compared to medium with 20% KnockOut serum replacement, in which spontaneously-beating cardiomyocytes were only observed in the FBS-treated group.14) Moreover, bone morphogenetic protein 4 (BMP-4) was not shown to induce cardiomyocyte differentiation in mouse ESCs with serum-free models; at least a small amount of FBS in the hanging drop stage is necessary. These findings suggest that serum factors are not critical after the initial activation, but do enhance the differentiation of cardiomyocytes.15) By contrast, the number of beating areas in the co-culture model with END-2 cells was increased 24-fold in the absence of fetal calf serum (FCS) compared to the cells in the presence of 20% FCS.16) These results suggest that pro- and anti-cardiogenic factors may be present in the serum. The stimulatory or inhibitory effects of the serum concentration on cardiac differentiation may be different in various stem cell lines and culture conditions.
Cardiomyogenic differentiation of P19 cells has generally been induced with the combination of EB formation and DMSO in bacterial dishes.7) However, we previously reported that 5-azacytidine induced cardiomyogenic differentiation of P19 cells in confluent monolayer cultures without prior EB formation and DMSO exposure via, in part, activation of BMP signaling molecules.9) In this context, we investigated whether 5-azacytidine could enhance cardiomyogenic differentiation of P19 cells at the stage of preformed EB in the presence of DMSO in bacterial dishes. Our results showed that 1 µM 5-azacytidine-treated cells had the most enhanced cardiomyogenic differentiation of P19 cells, both under low-serum culture conditions (2% FBS) and high-serum culture conditions (10% FBS), compared to the 5-azacytidine non-treated control group and the 3 µM 5-azacytidine-treated group. This observation is consistent with the findings of our previous report9) that P19 cells differentiated into the cardiomyogenic cell lineage in response to 1 µM 5-azacytidine in a confluent monolayer culture. Various concentrations of 5-azacytidine with different treatment periods have been used to induce cardiomyogenic differentiation of mesenchymal stem cells derived from different species. Murine bone marrow stromal cells were treated with 3 µM 5-azacytidine for 24 hours, and spontaneously-beating cells were observed after 2 weeks.11) Mesenchymal stem cells isolated from adult human bone marrow were reported to differentiate to a cardiomyogenic lineage in vitro after treatment with 10 µM 5-azacytidine for 24 hours.17) Adult mesenchymal stem cells isolated from the fatty tissue of New Zealand White rabbits were treated with 1, 3, 6, 9, and 12 µM 5-azacytidine and incubated for 12, 24, 48, and 72 hours.18) Taken together, our results and previous reports suggest that the optimal concentration and treatment time with 5-azacytidine for the induction of cardiomyogenic differentiation appears to depend on both the efficacy of cardiac differentiation and the cytotoxicity of the stem cells.
In conclusion, the results of this study demonstrated that cardiomyogenic differentiation of P19 cells could be enhanced by a combination of different experimental factors, including the concentration of the serum and 5-azacytidine, as well as the culture time.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download