Abstract
Background and Objectives
Several studies have shown that angiotensin II receptor blockers (ARBs) improve endothelial function and arterial stiffness. Telmisartan is a highly selective ARB that activates peroxisome proliferator-activated receptor γ (PPARγ). The purpose of this study was to evaluate the effects of telmisartan, such as endothelial function, arterial stiffness, and insulin sensitivity, in patients with essential hypertension.
Subjects and Methods
Thirty-nine patients with essential hypertension were administered telmisartan (80 mg once daily) using an open-labeled and prospective protocol. The patients were examined before and 8 weeks after treatment to assess changes in flow mediated-vasodilation (FMD), pulse wave velocity (PWV), quantitative insulin-sensitivity check index (QUICKI), homeostasis model assessment (HOMA), and adiponection.
Results
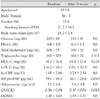
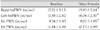
The systolic and diastolic blood pressure (BP) decreased from 153±15 mmHg and 90±13 mmHg to 137±16 mmHg and 84±10 mmHg after telmisartan treatment, respectively (p<0.01). Telmisartan therapy increased the FMD from 7.6±3.5 to 9.0±2.8% (p<0.01). The following parameters of arterial stiffness were significantly improved after telmisartan therapy: brachial-ankle pulse wave velocity (baPWV), from 17.2±3.1 to 15.9±2.6 m/sec; heart-carotid PWV (hcPWV), from 9.7±1.8 to 9.0±1.9 m/sec; and heart-femoral PWV (hfPWV), from 11.3±1.9 to 10.7±1.9 m/sec (p<0.01). There were no changes in QUICKI, the HOMA level, and plasma adiponectin (p=NS).
Hypertension is accompanied by vascular endothelial dysfunction, the underlying etiology of atherosclerosis, and hypertension is closely associated with metabolic disorders, such as obesity and type 2 diabetes mellitus, which are caused by insulin resistance.1)
The function of vascular endothelial cells is measured by flow-mediated, endothelium-dependent vasodilation (FMD) of the forearm. Vascular endothelial cells are examined and serve as useful indicators in the diagnosis and treatment of atherosclerosis and cardiovascular diseases. In patients who have risk factors for developing cardiovascular diseases, such as hypertension and diabetes, it is also well-known that dysfunction of vascular endothelial cells occurs due to the decreased vasodilation response.2) Arterial stiffness is also an indicator that can be used to predict cardiovascular risk factors. In recent years, measurement of the pulse wave velocity (PWV) has frequently been performed to predict major cardiovascular events.3)4) Because arterial stiffness is increased in patients with hypertension, the PWV is decreased, i.e., the improvement in arterial stiffness is used to determine the additional effects of anti-hypertensive drugs.5)6)
Previous studies have shown that angiotensin converting enzyme (ACE) inhibitors or statins have a vasodilatory effect based on the improvement in vascular endothelial function.6-8) It has also been reported that some angiotensin II type 1 receptor blockers (ARBs) improve vascular endothelial function by activation of peroxisome proliferator-activated receptor γ (PPARγ) via anti-inflammatory action and an anti-oxidative effect. Furthermore, ARBs have also been reported to elevate adiponectin, which is associated with an improvement in insulin resistance.9-12) In Korea, however, few studies have examined the effects of ARBs and telmisartan on vascular endothelial function, arterial stiffness, and insulin sensitivity.12)21)26)
In the current study, we examined the effects of ARB and telmisartan on vascular endothelial function, arterial stiffness, and insulin resistance in patients with essential hypertension.
The current study was conducted in 39 patients with less than severe hypertension (systolic pressure <180 mmHg or diastolic pressure <110 mmHg) who were evaluated in the Department of Cardiology of our hospital. These patients consisted of 36 men and 3 women, with a mean age of 61±6 years. The current study was approved by the Institutional Review Board (IRB) of our hospital. All of the patients were given a full explanation of the study objectives before signing a written informed consent. Exclusion criteria were severe hypertension, acute coronary syndrome, cardiac failure, renal failure, liver cirrhosis, and uncontrolled diabetes {hemoglobin A1c (HbA1c) >10.0%}.
At the time of the baseline visit, the patients underwent the following laboratory tests: general blood test, serum biochemistry, EKG, chest radiography, and urinalysis. Following a 2-week screening period, the current study was initiated. Following a 2-week wash-out period in patients who were taking anti-hypertensive drugs, such as statins or ARBs, and after the screening period, telmisartan (80 mg) was administered. Prior to the administration and at week 8, blood tests, FMD, PWV, and indices of insulin resistance, such as quantitative insulin sensitivity check index (QUICKI) and homeostasis model assessment (HOMA and adiponectin), were compared.
For blood pressure measurement, stabilization was attempted for >10 minutes and the blood pressure was measured 2 times at a minimum interval of 10 minutes in the right forearm in a sitting position. Then, the measurements were averaged. Cases in which the systolic pressure was >140 mmHg or the distolic pressure was >90 mmHg were defined as hypertension.
The evaluation of vascular endothelial function was performed by FMD, a non-invasive method. After an more than 8-hour fast, patients were stabilized in the supine position in a quiet place where the indoor temperature was maintained at 22-23℃ and the inferior part of their right forearm was placed in a blood pressure cuff. Following this, the FMD measurement was performed. To assure that the ultrasonographic findings of the humeral artery were detected, the most accessible area which was 2-5 cm inferior to the antecubital fossa was targeted by a high-resolution ultrasonography unit (Sequoia 512; Acuson, USA), to which a 10 MHz lineararray transducer was implanted, and ultrasonography was performed according to the methods that have been reported in a prior publication.13)14) The baseline diameter of the brachial artery was measured, and the pressure of a barometer which surrounded the arm was elevated to 200 mmHg. The blood flow in the brachial artery was blocked for 5 minutes. Then, the pressure was abruptly dropped to 0 mmHg and the vasodiliatory response was measured 3 times for 1 minute. In measuring the diameter of the blood vessels, the homogeneous images of the intima could not be obtained. The distance between the media, corresponding to the tunica media, was alternatively measured. To minimize the error in the measurements, the possible baseline point was established in the areas where the arterial branch was placed. The time point of measurement was the endpoint of the diastole phase. Thus, the diameter was measured immediately before the initial part of the R wave on the EKG. Following the analysis of the change in diameter, using the diameter which was maximally extended, the increased diameter compared to the baseline measurement was calculated as a percentile value (%). Thus, the degree of vasodilation was obtained {FMD %=(maximum dilated diameter-baseline diameter)/baseline diameter×100}.
Blood sampling was done in the morning prior to the treatment and following 8-week of drug administration an more than 8-hour fasting. Plasma insulin was measured with a radioimmunoassay (RIA; Biosource Inc., Nivelles, Belgium) and adiponectin was measured with a RIA (LINCO Research Inc., Missouri, USA). Indices for insulin sensitivity (QUICKI and HOMA) were calculated based on the following formula: QUICKI=1/{log (insulin)+log (glucose)} and HOMA=fasting insulin×fasting glucose/22.5.
The units of insulin and glucose were µU/mL and mg/dL, respectively.15)
PWV was measured based on conventional methods with the use of autowave form analysis (VP-2000; Colin Medical Technology Co., Komaki, Japan) in a fasting state following stabilization of the heart rate.16) The pulse wave velocity between the bilateral brachial arteries and the ankle (ba) was measured by placing both arms and the ankle in a cuff, to which an oscillometric sensor was implanted. A tonometric sensor was placed in the heart-femoral (hf) and the heart-carotid (hc) for recording.
During the trial, the occurrence of side effects was confirmed by physical examination, as well as hematologic measures.
All data are expressed as the mean±SD. A comparison of the measurements prior to and following administration was made using a paired t-test. All the statistical procedures were performed using Statistical Package for Social Science (SPSS) 13.0 (SPSS Inc., Chicago, IL, USA). A p<0.05 was considered statistically significant.
Of 42 patients who took drugs for 8 weeks, 39 patients had a follow-up visit. Of the three patients who were dropped from the current analysis, two did not visit the outpatient clinic and the remaining patient declined to take the test. The subject patients consisted of 36 males and 3 females, with a mean age of 61±6 years (Table 1). Twelve patients had type 2 diabetes mellitus. In these patients, the mean weight was 69.8±8.2 kg and the body mass index (BMI) was 24.7±2.4 gm/m2. Neither hypotension nor hyperkalemia occurred in any of the patients. Likewise, discontinuation of drug treatment did not occur. In the subject patients, the sitting position blood pressure was measured following the administration of telmisartan. This showed that the systolic pressure was significantly decreased from 153±15 mmHg to 137±16 mmHg (p<0.01) and the diastolic pressure was significantly decreased from 90±13 mmHg to 84±10 mmHg (p<0.01; Fig. 1). On blood testing, there were no significant differences in cholesterol, triglycerides, and low-density lipoprotein- cholesterol. The concentration of high-density lipoprotein cholesterol was significantly increased (51±12 vs. 53±17 mg/dL, p<0.01). However, there were no significant differences in high sensitivity C-reactive protein (hs-CRP) (1.9±2.9 vs. 1.8±2.5 mg/dL, p=NS), hemoglobin A1c (HbA1c) (6.4±1.0 vs. 6.3±1.2%, p=NS), and N-terminal pro-B-type natriuretic peptide (NT-proBNP) (39.1±45.2 vs. 26.7±26.6 pg/dL, p=0.063; Table 1).
Vasodilatory responses were improved from 7.6±3.5% at baseline to 9.0±2.8% following the administration of telmisartan (p<0.01; Fig. 2). The PWV was significantly decreased prior to the administration of telmisartan and following the administration of telmisartan between the bilateral brachial and ankle artery and between the heart and the carotid artery (Table 2). However, there were no significant difference in QUICKI (0.36±0.04 vs. 0.37±0.04, p=0.083), HOMA (2.16±1.63 vs. 1.78±1.33, p=NS), and adiopnectin (7116.3±2379.8 vs. 7795.6±3815.7 µg/mL, p=NS).
In the current study, telmisartan was administered at a dose of 80 mg/day for 8 weeks in patients with essential hypertension. It was shown that the sitting systolic and diastolic pressures were significantly decreased and the function of vascular endothelial cells and arterial stiffness were improved.
It has been well-demonstrated that angiotension II plays a crucial role in the gradual progression of tissue injury and atherosclerosis in structures, such as blood vessels, kidneys, heart, and brain.10) There exists an abundance of results regarding the long-term protective effect of ARBs in inhibiting the activity of angiotensin II. According to the ONTARGET study, the results of which have been reported in recent years, ARBs have been demonstrated to have a preventive effect for cardiovascular events equivalent to ramipril.17) Presumably, these results might be due to the very strong activity of PPARγ as well as the high histocompatibility and very long half-life of telmisartan.17)18)
Vascular endothelial cells are mainly responsible for the progression of atherosclerosis and the regulatory process of vascular stiffness, which are known to be damaged by various risk factors of developing atherosclerosis.2) Some authors have attempted to improve the function of vascular endothelial cells with the use of anti-hypertensive drugs. Accordingly, it has been shown that ACE inhibitors and ARBs increase the endothelium-dependent vasodilatory responses.2)10)19) It has also been reported that ARBs suppress the arterial damage and the atherosclerotic progression due to hypercholesterolemia and hypertension.20) In the current study, it was first demonstrated that the administration of telmisartan (80 mg) significantly increased the vasodilatory response during an 8-week period in Korea. It can eventually be inferred that the administration of telmisartan will have an affirmative effect on the progression of atherosclerosis due to the improvement of vascular endothelial functions. These findings are in agreement with the report of Cho et al.,19) which showed that ramipril had a short-term effect on the improvement of vascular endothelial function. The inhibition of the activation of the renin-angiotensin system can be considered as the key mechanism by which atherosclerotic progression is suppressed. In the current study, however, the effect of the long-term administration of telmisartan remains unclear. Because the current study was conducted in the absence of a control group, it was also unclear whether the vascular endothelial function were improved by the anti-hypertensive effect or occurred as a result of the drug effect itself. Considering the previous studies conducted by Koh et al. showing that pioglitazone (a PPARγ activator) improved the function of vascular endothelial cells in patients with metabolic syndrome; however, it is assumed that the effect of telmisartan itself could not be completely ruled out.11)12)
With respect to the metabolic effect, it has been shown that ARBs have a positive effect on the occurrence of diabetes mellitus by increasing the insulin-mediated absorption of glucose, stimulating adipogenesis, inducing the differentiation of adipocytes, and activating PPARγ. This has also been demonstrated in a large-scale clinical trial.22) This effect has been reported to have a relationship with the activation of PPARγ with no association to the degree of blocking of the angiotension II type 1 receptor.23) In particular, telmisartan has the most powerful effect in activating PPARγ. Accordingly in the current study, the effect of telmisartan on insulin sensitivity was expected. Indices for insulin sensitivity, including QUICKI, HOMA, and adiponectin, were measured. This showed that the administration of telmisartan did not improve insulin sensitivity. Presumably, this might be because a majority of the subject patients included patients with hypertension who were different from those with metabolic syndrome or diabetes mellitus and who concurrently had a severe metabolic disorder. A rigorous management of diet and exercise therapy was not simultaneously instituted. The current study was conducted during a relatively short, 8-week period. Despite a lack of statistical significance, however, the overall parameters were improved. A persistent treatment would therefore produce more positive outcomes.
It is generally known that all the anti-hypertensive drugs do not reduce arterial stiffness. Calcium blockers or ACE inhibitors are known to be effective in this series. In the current study, arterial stiffness was significantly reduced. This was greatly different from the previous studies, conducted by Rhee at al.,6) reporting that stiffness of the aorta was not improved by the administration of losartan. Presumably, this might be due to the differences in the patient group or the administration dose {losartan (50 mg) vs. telmisartan (80 mg)}. As compared with the previous small-scale studies which were conducted using ARBs in patients with essential hypertension, despite the differences in the period of drug administration, dose, the number of enrolled patients, and the type of concomitant drugs, the current study showed that the vasodilatory responses, PWV, and insulin resistance were significantly improved or showed a tendency toward improvement.6)24-26) The results of the current study cannot be understated, but it can be inferred that telmisartan has its own effect on the improvement of vascular endothelial functions and arterial stiffness from the aspect of the class effects of angiotension II inhibitors and insulin sensitivity.
The limitations of the current study were as follows:
1) The current study was conducted in a relatively small number of enrolled patients, and it was conducted in the absence of a control group. Further large-scale studies are therefore warranted to obtain more significant results.
2) The current study was conducted without a rigorous management of living habits, such as exercise or dietary habit. Blood glucose control or laboratory results might affect the results of the current study. During the conduct of a trial, however, the types of drugs were not changed. The changes in the functions of vascular endothelial cells or PWV might originate from the additional effects of ARB rather than their anti-hypertensive ones, as shown in other studies.10)27)
In conclusion, the short-term administration of telmisartan significantly improved the functions of vascular endothelial cells and the arterial stiffness in patients with less than severe essential hypertension. But insulin sensitivity was not improved.
Figures and Tables
Fig. 1
Effects of telmisartan on systolic and diastolic blood pressure (BP). P<0.05 vs. baseline value.

Fig. 2
Change of flow mediated-dilation (FMD) at baseline and 8 weeks after telmisartan treatment. P<0.01 vs. baseline value.

References
1. Han SH, Quon MJ, Koh KK. Reciprocal relationships between abnormal metabolic parameters and endothelial dysfunction. Curr Opin Lipidol. 2007. 18:58–65.
2. On YK, Chung WY, Kim YS, et al. Improvement in endothelial function by angiotensin-converting enzyme inhibition and vitamin C in essential hypertension. Korean Circ J. 2001. 31:411–419.
3. Chalmers J, MacMahon S, Mancia G, et al. 1999 World Health Organization-International Society of Hypertension Guidelines for the management of hypertension: guidelines subcommittee. Clin Exp Hypertens. 1999. 21:1009–1060.
4. Blacher J, Asmar R, Djane S, London GM, Safar ME. Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension. 1999. 33:1111–1117.
5. Safar ME, Toto-Moukouo JJ, Bouthier JA, et al. Arterial dynamics, cardiac hypertrophy, and antihypertensive treatment. Circulation. 1987. 75:I156–I161.
6. Rhee MY, Han SS, Lyu S, Lee MY, Kim YK, Yu SM. Short-term treatment with angiontensin II antagonist in essential hypertension: effects of losartan on left ventricular diastolic dysfunction, left ventricular mass, and aortic stiffness. Korean Circ J. 2000. 30:1341–1349.
7. Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med. 2000. 342:145–153.
8. Demers C, McMurray JJ, Swedberg K, et al. Impact of candesartan on nonfatal myocardial infarction and cardiovascular death in patients with heart failure. JAMA. 2005. 294:1794–1798.
9. Bahadir O, Uzunlulu M, Oguz A, Bahadir MA. Effects of telmisartan and losartan on insulin resistance in hypertensive patients with metabolic syndrome. Hypertens Res. 2007. 30:49–53.
10. Morimoto S, Yano Y, Maki K, Sawada K. Renal and vascular protective effects of telmisartan in patients with essential hypertension. Hypertens Res. 2006. 29:567–572.
11. Koh KK, Quon MJ, Han SH, et al. Vascular and metabolic effects of combined therapy with ramipril and simvastatin in patients with type 2 diabetes. Hypertension. 2005. 45:1088–1093.
12. Koh KK, Quon MJ, Han SH, Chung WJ, Lee Y, Shin EK. Anti-inflammatory and metabolic effects of candesartan in hypertensive patients. Int J Cardiol. 2006. 108:96–100.
13. Cho SH, Park IH, Jeong MH, et al. Increased inflammatory markers and endothelial dysfunction are associated with variant angina. Korean Circ J. 2007. 37:27–32.
14. Kim W, Jeong MH, Cho SH, et al. The effect of green tea on endothelial function and the circulating endothelial progenitor cell in chronic smokers. Korean Circ J. 2006. 36:292–299.
15. Katz A, Nambi SS, Mather K, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000. 85:2402–2410.
16. Kim YK, Lee MY, Rhee MY. A simple oscilometric measurement of pulse wave velocity: comparison with conventional tonometric measurement. Korean J Med. 2004. 67:597–606.
17. Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008. 358:1547–1559.
18. Benson SC, Pershadsingh HA, Ho CI, et al. Identification of telmisartan as a unique angiotensin II receptor antagonist with selective PPARγ-modulating activity. Hypertension. 2004. 43:993–1002.
19. Cho BK, Park KR, Kim KY, Bae JH. Effects of ramipril on vascular response in patients with coronary artery disease. Korean Circ J. 2002. 32:674–679.
20. Strawn WB, Chappell MC, Dean RH, Kivlighn S, Ferrario CM. Inhibition of early atherogenesis by losartan in monkeys with diet-induced hypercholesterolemia. Circulation. 2000. 101:1586–1593.
21. Kho JS, Park SJ, Im SI, Choi BR, Kwak CH, Hwang JY. Peroxisome proliferators-activated receptor gamma agonist improves endothelial function in diabetic patients with metabolic syndrome: pivotal role of NOx and inflammation. Korean Circ J. 2007. 37:221–229.
22. Yusuf S, Ostergren JB, Gerstein HC, et al. Effects of candesarta on the development of a new diagnosis of diabetes mellitus in patients with heart failure. Circulation. 2005. 112:48–53.
23. Schupp M, Janke J, Clasen R, Unger T, Kintscher U. Angiotensin type 1 receptor blockers induce peroxisome proliferators-activated receptor-γ activity. Circulation. 2004. 109:2054–2057.
24. Benndorf RA, Rudolph T, Appel D, et al. Telmisartan improves insulin sensitivity in nondiabetic patients with essential hypertension. Metabolism. 2006. 55:1159–1164.
25. Mori Y, Itoh Y, Tajima N. Telmisartan improves lipid metabolism and adiponectin production but does not affect glycemic control in hypertensive patients with type 2 diabetes. Adv Ther. 2007. 24:146–153.
26. Han SH, Lee SJ, Oh BC, Koh KK, Shin EK. The additive beneficial effects of ramipril combined with candesartan in hypertensive patients on insulin resistance, plasma adiponectin. Korean Circ J. 2007. 37:173–179.
27. Benndorf RA, Appel D, Maas R, Schwedhelm E, Wenzel UO, Böger RH. Telmisartan improves endothelial function in patients with essential hypertension. J Cardiovasc Pharmacol. 2007. 50:367–371.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download