Abstract
Anomalous origin of the right or left coronary artery from the contralateral sinus of Valsalva is often asymptomatic, but many patients, particularly young ones, present with sudden death or myocardial ischemia without symptoms. The mechanism of sudden death in this entity is unclear and has not been fully evaluated. These anomalies are rare, and many cardiologists and radiologists are unfamiliar with them. Surgical repair is recommended, especially with anomalous origin of the left coronary artery (LCA). However, there is controversy concerning the treatment of anomalous right coronary artery (RCA) with interarterial course due to its relatively high incidence and the fact that it leads to few, if any, clinical problems.
The incidence of coronary artery anomalies is approximately 1% among patients undergoing cardiac catheterization,1-4) 0.29% among autopsy specimens,5) and less than 0.1% among prospective echocardiographic screenings.6) Most anomalies are incidentally detected and do not create clinical problems.1-4) However, 19-33% of sudden cardiac deaths in the young population are attributable to coronary artery anomalies.7-9) An anomalous origin of the right coronary artery (RCA) from the left sinus is a very rare anomaly, and its incidence is 0.019% to 0.49% on coronary angiography.1-4) However, recent angiographic studies have reported a relatively high incidence (5.6%) of coronary artery anomalies and anomalous RCA origins from the left sinus (0.92%).10) Anomalous origin of the RCA (0.17%) is more common than anomalous origin of left coronary artery (LCA, 0.047%).1) Coronary artery anomalies are classified as benign (80.6%) but potentially serious anomalies (19.4%). Potentially serious anomalies include ectopic origin from the pulmonary artery, ectopic origin from the opposite aortic sinus, single coronary artery, and large coronary fistulae.1) These anomalies may be associated with sudden death.
Anomalous origin of either the right or left main coronary artery from the contralateral sinus with an interarterial course between the aorta and the pulmonary trunk may also be associated with sudden death and may cause myocardial ischemia, arrhythmia, and syncope.11-19) There have been several reports concerning these anomalies and their attendant clinical problems in Korea.20-25) The incidence of anomalous RCA is higher in angiographic studies and lower in autopsy studies compared to that of anomalous LCA, which is also a more common cause of sudden death (Table 1).11-17) Anomalous LCA is more common in young male subjects,11-17) possibly because of the longer intramural course and larger area of dependent myocardium in the left ventricle.26)27) The incidence of anomalous RCA from the left coronary sinus differs among the races: the incidence in Western countries is 27%, and the incidence in Japan is 79% (44 of 56 patients). Death has not been reported in relation to this anomaly in any of the 44 Japanese patients. An interarterial course existed in 12 anomalous LCA cases.17) Study of the proximal structures, including the takeoff portion, is important in formulating a treatment plan,3)10-19)26)28-30) and many imaging tools are now available to evaluate this anomaly. Multi-detector CT (MDCT) is the favored imaging method. This review describes the pathophysiology, imaging methods, surgical methods, and treatment of anomalous RCA from the left coronary sinus with interarterial course.
The pathophysiology of the restricted coronary blood flow seen in this anomaly is suggested to be as follows. The acute takeoff angle, slit-like orifice, and compression of the intramural segment by the aortic valve commissure are all thought to narrow the orifice. Lateral luminal compression of the intramural portion of the coronary artery and compression of the coronary artery between the aorta and the pulmonary artery are also possible mechanisms (Fig. 1).11-19)28)31)32) Some autopsy-based studies have shown that slit-like orifice structure and acute angle takeoff are more common in sudden cardiac death patients.14-16) In a MDCT-based study, acute angle takeoff was correlated with luminal stenosis of the orifice.25) However, there is still controversy concerning the mechanism by which the interarterial course is compressed between the aorta and the pulmonary artery. An intravascular ultrasound (IVUS) study26) found that luminal compression of the coronary artery was totally attributable to the aorta because the pressure of the pulmonary artery was much lower than that of the aorta. Another MDCT study showed that the narrowing of the orifice was more severe than that of the interarterial course was.25) However, the sudden cardiac death associated with this anomaly is related to severe exercise, and our study was conducted at rest, so further mechanistic evaluation is necessary.
The correlation between coronary artery obstructive disease and coronary anomalies is uncertain. Some authors insist that anomalous coronary arteries increase the risk of coronary artery obstructive disease,33)34) but the prevailing opinion is that the anomalous portion of the coronary artery is not vulnerable to obstructive disease,4)29)35)36) The incidence of concomitant congenital anomalies is 4.2-24%; common anomalies include bicuspid aortic valve and mitral valve prolapse.3)25)
The methods used to evaluate anomalous RCAs include echocardiography, angiography, MDCT, and MRI. Noninvasive tools such as MDCT19)37-41) and MRI42-45) can provide precise information about the complex anatomy of coronary artery anomalies, though MDCT is favored due to its higher spatial resolution and rapid exam time. Additionally, MDCT can provide numerous multiplanar image reconstructions to permit precise evaluation of the takeoff portion and course of the anomalous coronary artery (Fig. 2). Transthoracic echocardiography provides limited information in this regard, and transesophageal echocardiography provides more information,22) but both of these methods are invasive. Evaluation is difficult in angiography because the complex three-dimensional structure of the anomalous coronary artery is displayed in a two-dimensional plane, and selective cannulation of the anomalous coronary artery is made difficult by the small, slit-like orifice. The success rate of selective cannulation is 55-61%.25)39)40)41) This success rate is not correlated with the takeoff angle or the orifice size or shape, so the relatively low Figure has been attributed to limited experience on the part of physicians, who rarely see this anomaly.25) MDCT-guided cannulation may be useful in increasing the success rate of cannulation. IVUS is also a useful method for obtaining cross-sectional luminal images,21) but cannulation is difficult, and this method is also invasive. MDCT provides excellent information concerning orifice location and the course of the anomalous coronary artery, so MDCT should be performed prior to angiography or IVUS. The size and shape of the slit-like orifice differ according to image projection, so multiplanar image reconstruction is necessary in precise evaluation. Angiography and echocardiography are invasive, have a relatively low cannulation success rate, and are limited with respect to multiplanar image reconstruction. Therefore, MDCT is the best method for imaging coronary artery anomalies despite its radiation. Further developments in MRI may lead to displacement of MDCT in the future.
Surgical treatment methods are variable. The unroofing procedure31)32) manipulates the orifice, enlarges the orifice, and makes an acute angulation, which decreases the lateral compression of the intramural segment. Possible serious complications of this procedure include aortic valve incompetence due to injury of the intercoronary commissure. This method does not manipulate the interarterial course and has good results. Associated findings support the suggestion that the pulmonary artery has little or no effect on the constriction of coronary blood flow. Percutaneous coronary intervention (PCI)20)46)47) relieves systolic compression, but selective cannulation and stent insertion in the anomalous RCA are difficult to perform due to the small, ectopic orifice and the long, curved intramural portion of the anomalous RCA. Prior to PCI, the anomalous RCA must be evaluated by MDCT; MDCT-guided cannulation is helpful in selective cannulation and intervention.20)21) Coronary artery bypass grafting (CABG)32)48) is technically easy because it does not entail opening of the aorta or manipulation of the intercoronary commissure. However, the native anomalous RCA is patent at rest, so obstruction of the CABG by competition flow is possible.32) Ligation of the native anomalous RCA proximal to the anastomosis is a feasible method for preventing competition flow.48) Coronary reimplantation32) of the anomalous RCA in the right coronary sinus is also useful, but the disadvantage of this method is that it carries the risk of neoostial stenosis.
Sudden death without symptoms occurs frequently in patients with anomalous LCAs, so surgical repair is recommended.16)49) However, sudden death is extremely rare in asymptomatic patient with anomalous RCAs, and there is no sudden death in children under 10 years of age or adults over 30 years of age.49) Eckart et al.9) reported 21 coronary artery anomalies related to sudden death among 6,300,000 military recruits, and all cases were anomalous LCAs from the right coronary sinus. According to the MDCT-based study of Lee et al.,25) significant stenosis (>50%) of an anomalous RCA occurred in only 1 of 24 patients, and this patient, whose symptoms disappeared after an unroofing procedure (Fig. 3), now has an outstanding acute takeoff angle and a small orifice. Other patients with more obtuse angles and mild or absent narrowing of the orifice and artery exhibit no anomaly-related problems in the absence of treatment (Fig. 2). One report has suggested that subclinical ischemic changes in the myocardium are relatively frequent (8 of 16 patients) in anomalous RCA patients in the postoperative period.50) Treatment of anomalous RCA with an interarterial course from the left coronary sinus is still debated because most anomalous RCAs are benign, with a small risk of sudden death and late myocardial ischemia after surgery is undertaken. Pelliccia7) insists on treatment as follows. Young patients (<35 years) with symptoms or ischemia should undergo surgery. In young patients (<35 years) without symptoms or ischemia, the best therapy is uncertain. Older patients without symptoms or ischemia do not need surgical therapy. Strenuous exercise should be limited. Gersony49) suggests that anomalous RCAs should be followed without intervention and believes that the benefit of excessive exercise limitation is doubtful. I agree with this suggestion and believe most patients should be followed without aggressive treatment. If a young, symptomatic patient has significant luminal narrowing on imaging studies, surgical intervention should be considered.
Multiplanar MDCT image reconstruction at the takeoff portion of the anomalous RCA permits precise evaluation of the takeoff angle, size of the slit-like orifice, and course of the anomalous RCA. Most anomalous RCAs with interarterial courses from the left coronary sinus are benign. Precise and thorough imaging evaluation of anomalous RCAs is necessary prior to selection of treatment method.
Figures and Tables
Fig. 1
Possible mechanisms of coronary flow restriction of the anomalous right coronary artery (RCA) from the left coronary sinus with interarterial course. The intramural course of the anomalous RCA is long, and the takeoff angle of the RCA orifice is acute (★) compared with that of the normal left main coronary artery (LM). A combined slit-like orifice (arrow) is also seen. If the anomalous RCA passes through the aortic commissure (black column), compression by the aortic commissure (open arrow head) is also possible. Compression of the anomalous RCA by the pulmonary artery and aorta (open arrows) is also feasible.
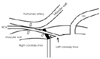
Fig. 2
A 68-year-old man presented with intermittent chest pain. Oblique axial MPR imaging (A) shows luminal narrowing at the takeoff portion of the anomalous RCA (arrow), but the oblique sagittal MPR (B) image shows a normal-sized anomalous RCA (arrow). Oblique coronal MPR imaging (C) shows an interarterial course; the lumen of the anomalous RCA is ovoid (arrow). This patient required no treatment. MPR: multiplanar reconstruction, RCA: right coronary artery, PA: pulmonary artery, Ao: aorta, LCC: left coronary sinus, RCC: right coronary sinus.

Fig. 3
A 39-year-old woman presented with persistent chest pain and palpitations. Angiography (A) showed an anomalous RCA (arrow) originating from the left coronary sinus, but selective cannulation failed due to the small orifice and angulation. Preoperative oblique axial (B) and curved (C) MPR images showed severe luminal narrowing (arrow) of the proximal portion of the anomalous RCA, as well as a small orifice (arrow head) with acute angle takeoff. After an unroofing procedure was performed, postoperative oblique (D) and curved (E) MPR images showed a more distended proximal anomalous RCA (arrow) and orifice (arrow head) with increased takeoff angle. The unroofing procedure manipulates the orifice and the intramural portion of the anomalous RCA and does not manipulate the interarterial course. This case was previously reported elsewhere.19) PA: pulmonary artery, Ao: aorta, MPR: multiplanar reconstruction, RCA: right coronary artery.
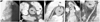
References
1. Yamanaka O, Hobbs RE. Coronary artery anomalies in 126,595 patients undergoing coronary arteriography. Cathet Cardiovasc Diagn. 1990. 21:28–40.
2. Kardos A, Babai L, Rudas L, et al. Epidemiology of congenital coronary artery anomalies: a coronary arteriography study on a central European population. Cathet Cardiovasc Diagn. 1997. 42:270–275.
3. Topaz O, DeMarchena EJ, Perin E, Sommer LS, Mallon SM, Chahine RA. Anomalous coronary arteries: angiographic findings in 80 patients. Int J Cardiol. 1992. 34:129–138.
4. Garg N, Tewari S, Kapoor A, Gupta DK, Sinha N. Primary congenital anomalies of the coronary arteries: a coronary: arteriographic study. Int J Cardiol. 2000. 74:39–46.
5. Alexander RW, Griffith GC. Anomalies of the coronary arteries and their clinical significance. Circulation. 1956. 14:800–805.
6. Pelliccia A, Spataro A, Maron BJ. Prospective echocardiographic screening for coronary artery anomalies in 1,360 elite competitive athletes. Am J Cardiol. 1993. 72:978–979.
7. Pelliccia A. Congenital coronary artery anomalies in young patients: new perspectives for timely identification. J Am Coll Cardiol. 2001. 37:598–600.
8. Maron BJ, Thompson PD, Puffer JC, et al. Cardiovascular preparticipation screening of competitive athletes: a statement for health professionals from the Sudden Death Committee (clinical cardiology) and Congenital Cardiac Defects Committee (cardiovascular disease in the young), American Heart Association. Circulation. 1996. 94:850–856.
9. Eckart RE, Scoville SL, Campbell CL, et al. Sudden death in young adults: a 25-year review of autopsies in military recruits. Ann Intern Med. 2004. 141:829–834.
10. Angelini P. Coronary artery anomalies--current clinical issues: definitions, classification, incidence, clinical relevance, and treatment guidelines. Tex Heart Inst J. 2002. 29:271–278.
11. Cheitlin MD, De Castro CM, McAllister HA. Sudden death as a complication of anomalous left coronary origin from the anterior sinus of Valsalva, a not-so-minor congenital anomaly. Circulation. 1974. 50:780–787.
12. Liberthson RR, Dinsmore RE, Fallon JT. Aberrant coronary artery origin from the aorta: report of 18 patients, review of literature and delineation of natural history and management. Circulation. 1979. 59:748–754.
13. Roberts WC, Siegel RJ, Zipes DP. Origin of the right coronary artery from the left sinus of Valsalva and its functional consequences: analysis of 10 necropsy patients. Am J Cardiol. 1982. 49:863–868.
14. Kragel AH, Roberts WC. Anomalous origin of either the right or left main coronary artery from the aorta with subsequent coursing between aorta and pulmonary trunk: analysis of 32 necropsy cases. Am J Cardiol. 1988. 62:771–777.
15. Frescura C, Basso C, Thiene G, et al. Anomalous origin of coronary arteries and risk of sudden death: a study based on an autopsy population of congenital heart disease. Hum Pathol. 1998. 29:689–695.
16. Taylor AJ, Rogan KM, Virmani R. Sudden cardiac death associated with isolated congenital coronary artery anomalies. J Am Coll Cardiol. 1992. 20:640–647.
17. Kaku B, Shimizu M, Yoshio H, et al. Clinical features of prognosis of Japanese patients with anomalous origin of the coronary artery. Jpn Circ J. 1996. 60:731–741.
18. Reul RM, Cooley DA, Hallman GL, Reul GJ. Surgical treatment of coronary artery anomalies: report of a 37 1/2-year experience at the Texas Heart Institute. Tex Heart Inst J. 2002. 29:299–307.
19. Kim CK, Park CB, Jin U, Lee BY, Song KS. Evaluation of unroofing procedure of anomalous origin of right coronary artery from left sinus of Valsalva between aorta and pulmonary trunk by multidetector computed tomography. J Comput Assist Tomogr. 2005. 29:752–755.
20. Kim JY, Yoon SG, Doh JH, et al. Two cases of successful primary percutaneous coronary intervention in patients with an anomalous right coronary artery arising from the left coronary cusp. Korean Circ J. 2008. 38:179–183.
21. Moon JY, Jeong CJ, Cho JY, et al. Anomalous origin of a right coronary artery with extrinsic compression between the great vessels: the intravascular ultrasound images. Korean Circ J. 2008. 38:390–392.
22. Cha KS, Kim HK, Cun KJ, et al. Role of transesophageal echocardiography in indentifying anomalous origin and course of coronary arteries. Korean Circ J. 1998. 28:576–585.
23. Cho HO, Cho KH, Jeong YS, et al. Anomalous origin of the left coronary artery from the right sinus of Valsalva, which presented as acute myocardial infarction. Korean Circ J. 2006. 36:817–819.
24. Kim HJ, Kim DK, Won JI, et al. Acute inferior wall myocardial infarction as a result of anomalous origin of the right coronary artery from the left sinus of Valsalva. Korean Circ J. 1997. 27:774–779.
25. In : ASCI 2008; –SE36. Abstract.
26. Angelini P, Velasco JA, Ott D, Khoshnevis GR. Anomalous coronary artery arising from the opposite sinus: descriptive features and pathophysiologic mechanisms, as documented by intravascular ultrasonography. J Invasive Cardiol. 2003. 15:507–514.
27. Taylor AJ, Byers JP, Cheitlin MD, Virmani R. Anomalous right or left coronary artery from the contralateral coronary sinus: "high-risk" abnormalities in the initial coronary artery course and heterogeneous clinical outcomes. Am Heart J. 1997. 133:428–435.
28. Virmani R, Chun PK, Goldstein RE, Robinowitz M, McAllister HA. Acute takeoffs of the coronary arteries along the aortic wall and congenital coronary ostial valve-like ridges: association with sudden death. J Am Coll Cardiol. 1984. 3:766–771.
29. Angelini P, Velasco JA, Flamm S. Coronary anomalies: incidence, pathophysiology, and clinical relevance. Circulation. 2002. 105:2449–2454.
30. Bloomfield P, Erhlich C, Folland ED, Bianco JA, Tow DE, Parisi AF. Anomalous right coronary artery: a surgically correctable cause of angina pectoris. Am J Cardiol. 1983. 51:1235–1237.
31. Garcia-Rinaldi R, Sosa J, Olmeda S, Cruz H, Carballido J, Quintana C. Surgical treatment of right coronary arteries with anomalous origin and slit ostium. Ann Thorac Surg. 2004. 77:1525–1529.
32. Romp RL, Herlong JR, Landolfo CK, et al. Outcome of unroofing procedure for repair of anomalous aortic origin of left or right coronary artery. Ann Thorac Surg. 2003. 76:589–595.
33. Kimbiris D. Anomalous origin of the left main coronary artery from the right sinus of Valsalva. Am J Cardiol. 1985. 55:765–769.
34. Jim MH, Siu CW, Ho HH, Miu R, Lee SW. Anomalous origin of the right coronary artery from the left coronary sinus is associated with early development of coronary artery disease. J Invasive Cardiol. 2004. 16:466–468.
35. Angelini P, Villason S, Chan AV Jr, Diez JG. Angelini P, editor. Normal and anomalous coronary arteries in humans. Coronary Artery Anomalies: A Comprehensive Approach. 1999. Philadelphia: Lippincott Williams & Wilkins;27–150.
36. Zhang F, Ge JB, Qian JY, Fan B, Wang QB, Chen HZ. Frequency of the anomalous coronary origin in the Chinese population with coronary artery stenosis. Zhonghua Nei Ke Za Zhi. 2005. 44:347–349.
37. Datta J, White CS, Gilkeson RC, et al. Anomalous coronary arteries in adults: depiction at multi-detector row CT angiography. Radiology. 2005. 235:812–818.
38. Kim SY, Seo JB, Do KH, et al. Coronary artery anomalies: classification and ECG-gated multi-detector row CT findings with angiographic correlation. Radiographics. 2006. 26:317–333.
39. Shi H, Aschoff AJ, Brambs HJ, Hoffmann MH. Multislice CT imaging of anomalous coronary arteries. Eur Radiol. 2004. 14:2172–2181.
40. Schmitt R, Froehner S, Brunn J, et al. Congenital anomalies of the coronary arteries: imaging with contrast-enhanced, multidetector computed tomography. Eur Radiol. 2005. 15:1110–1121.
41. van Ooijen PM, Dorgelo J, Zijlstra F, Oudkerk M. Detection, visualization and evaluation of anomalous coronary anatomy on 16-slice multidetector-row CT. Eur Radiol. 2004. 14:2163–2171.
42. Lee J, Choe YH, Kim HJ, Park JE. Magnetic resonance imaging demonstration of anomalous origin of the right coronary artery from the left coronary sinus associated with acute myocardial infarction. J Comput Assist Tomogr. 2003. 27:289–291.
43. Post JC, van Rossum AC, Bronzwaer JG, et al. Magnetic resonance angiography of anomalous coronary arteries: a new gold standard for delineating the proximal course? Circulation. 1995. 92:3163–3171.
44. Bunce NH, Lorenz CH, Keegan J, et al. Coronary artery anomalies: assessment with free-breathing three-dimensional coronary MR angiography. Radiology. 2003. 227:201–208.
45. White CS, Laskey WK, Stafford JL, NessAiver M. Coronary MRA: use in assessing anomalies of coronary artery origin. J Comput Assist Tomogr. 1999. 23:203–207.
46. Hariharan R, Kacere RD, Angelini P. Can stent-angioplasty be a valid alternative to surgery when revascularization is indicated for anomalous origination of a coronary artery fromthe opposite sinus? Tex Heart Inst J. 2002. 29:308–313.
47. Ceyhan C, Tekten T, Onbasili AO. Primary percutaneous coronary intervention of anomalous origin of right coronary artery above the left sinus of Valsalva in a case with acute myocardial infarction: coronary anomalies and myocardial infarction. Int J Cardiovasc Imaging. 2004. 20:293–297.
48. Shah AS, Milano CA, Lucke JP. Anomalous origin of the right coronary artery from the left coronary sinus: case report and review of surgical treatments. Cardiovasc Surg. 2000. 8:284–286.
49. Gersony WM. Management of anomalous coronary artery from the contralateral coronary sinus. J Am Coll Cardiol. 2007. 50:2083–2084.
50. Brothers JA, McBride MG, Seliem MA, et al. Evaluation of myocardial ischemia after surgical repair of anomalous aortic origin of a coronary artery in a series of pediatric patients. J Am Coll Cardiol. 2007. 50:2078–2082.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download